Bladder catheterization
is a standard procedure used for direct bladder drainage. Essentially, this is the insertion of a catheter into the bladder to remove urine from it, administer medications, take urine for examination or rinse the bladder. It is worth noting that catheterization is not a safe procedure for the patient and should be carried out if necessary and only by a specialist.
Bladder catheterization is performed for the following purposes:
Diagnostic goal:
obtaining urine samples to determine the microflora of the bladder and establish the cause of the disease
Therapeutic goal:
forced emptying of the bladder, lavage of the bladder, delivery of drugs to the site of inflammation
Hygiene goal:
caring for bedridden patients
Types of catheters
The most common in pediatric urological practice are Foley catheters. They differ in thickness, length, type of material, number of strokes, tail configuration and balloon size.
Thickness – caliber – is measured in Charrière units F=0.33 mm. The calibers of pediatric catheters are from 8 to 12 F, that is, from 2.64 to 3.96 mm. A catheter of a smaller diameter is used to ensure the outflow of urine, a larger one is used for rinsing and administering pharmaceuticals.
The material of the product is selected depending on the therapeutic purposes. Latex, plastic and PVC catheters are intended for short-term catheterization. For permanent catheterization, hydrogel, latex-silicone and silver-coated polymer models are used. Silicone catheters are placed in children with latex allergies.
If long-term urine drainage is required, catheters with several strokes and a balloon are used. The passages are needed to ensure the outflow of urine and to perform other therapeutic and diagnostic procedures. The balloon puts pressure on the bottom of the bladder, stimulating drainage and pressing on blood vessels during bleeding. The volume of the balloon for pediatric catheters is up to 2.5 to 5 ml.
For narrowing of the urinary tract, catheters with stylets - metal internal conductors - are used to facilitate insertion. A catheter with stylets is placed only by qualified urologists; all other catheters can be placed by a specially trained nurse.
Vesicoureteral reflux in children: therapeutic tactics
Among children and adolescents, vesicoureteral reflux (VUR) occurs in 1% of cases, the proportion of bilateral reflux is up to 50.9% [1]. Moreover, in 50% of cases the degree of reflux is different from different sides. The prevalence of PMR and the consequences of this disease (chronic pyelonephritis, developmental delay, arterial hypertension, chronic renal failure), inevitably leading to disability, dictate the need to find ways to improve treatment tactics. Among the causes of the development of VUR in boys, the main place is occupied by malformations of the ureteral orifices, while in girls, secondary forms of VUR, caused by urinary tract infection and neurogenic bladder dysfunction (NDBD), predominate. Thus, in the first year of life, the ratio of boys and girls suffering from PMR is 6:1, and as they grow older, this ratio changes exactly the opposite [2].
To understand the reasons for the development of VUR, it is necessary to have an idea of the normal structure of the vesicoureteral segment (VUS) (Fig.). Anatomically, the closing function of the PMS is carried out due to a certain ratio of the length and width of the intravesical section of the ureter (5:1), and the oblique passage of the ureter through the wall of the bladder. The long submucosal tunnel is a passive element of the ureterovesical “valve”. The active element of the valve mechanism is represented by the muscular-ligamentous apparatus of the ureter, which, when the detrusor contracts, closes the orifice.
The causes of antiphysiological urine flow include pathological conditions leading to disruption of the closure function of the PMS and high intravesical urine pressure. The first include congenital defects of the PMS and the inflammatory process of the superficial and deep layers of the triangles of the bladder (cystitis), which disrupts the functioning of the detrusor or the PMS itself.
PMS anomalies are a consequence of improper development of the ureteric outgrowth of the Wolffian duct at the 5th week of embryogenesis [3, 4]. PMS anomalies can be represented as follows:
- wide, constantly gaping shape of the ureteral orifice;
- location of the ureteric orifice outside the vesical triangle (lateroposition);
- complete absence or shortening of the submucosal section of the PMS;
- violation of the morphological normal structure of the PMS (dysplasia).
Loss of PMS closure function occurs when the bladder wall or PMS area becomes inflamed. Most often, secondary PMR is a consequence (complication) of granular, bullous or fibrinous forms of cystitis. Urinary tract infection occurs in 1–2% of boys and 5% of girls. More often, the urinary tract is colonized by opportunistic (intestinal) flora, among which Escherichia coli occupies the main place (40–70%) [3].
Normally, the PMS is able to withstand intravesical fluid pressure of up to 60–80 cm of water column [5]. High hydrostatic pressure is a consequence of intravesical obstruction or functional bladder disorders. Intravesical obstruction develops with posterior urethral valves, cicatricial phimosis in boys, sclerosis of the bladder neck (Marion's disease), and meatal stenosis in girls.
NDMPs occur in 20% of children aged 4–7 years. By the age of 14, the number of people suffering from these dysfunctions decreases to 2% [6]. NDMP manifest themselves as irritative or obstructive symptoms. The main forms of NDMP are: detrusor overactivity, detrusor hypotonia and detrusor-sphincter dyssynergia. In these conditions, VUR is also considered secondary and is a consequence of increased fluid pressure in the bladder. Detrusor overactivity is characterized by sharp jumps in intravesical pressure and impaired storage function of the bladder. Detrusor hypotension is characterized by a decrease in the sensitivity of the bladder wall, its overflow and an increase in urine pressure in its lumen above critical figures. Detrusor-sphincter dyssynergia is a violation of the synchronous functioning of the detrusor and sphincter apparatus, leading to functional bladder outlet obstruction during miction.
With age, there is a tendency towards a decrease in the incidence of primary and an increase in secondary VUR. In this case, the frequency of regression of primary VUR is inversely related to the degree of VUR. With I and II degrees of VUR, regression is observed in 80% of cases, and with III degrees - in only 40% of cases. An explanation for this is provided by the theory of “maturation” of PMS (Hutch, 1961), which acquired supporters later (Kellerman, 1967; King, 1974). The essence of the theory is that with the development of the child, a physiological transformation of the PMS occurs - the intravesical section of the ureter lengthens, its diameter decreases relative to its length, and the angle of entry into the bladder changes.
The modern treatment strategy for PMR includes a set of measures (therapeutic and surgical) aimed at eliminating the cause of reflux and eliminating its consequences. The choice of method for correcting VUR is, of course, determined by its form.
The essence of conservative therapy should be to eradicate urinary tract infections and eliminate functional disorders of the bladder and prevent the death of renal parenchyma. According to Yu. F. Isakov, the effectiveness of conservative therapy for grades I–III VUR is 60–70%. The main complications (manifestations) of PMR are chronic pyelonephritis (50–70%) and refluxogenic nephropathy (60–70%) [2]. The etiological structure of chronic pyelonephritis (according to A.F. Vozianov et al., 2002) is presented as follows: Escherichia coli - 40-60%, Proteus - 9-16%, Klebsiella - 7-20%, Streptococcus - 4-10%, L-forms - 15%, microbial associations - 10-15%, enterococcus - 2-5%, Pseudomonas aeruginosa - 2-7%, enterobacter - 5-15%, staphylococcus - 5-14%.
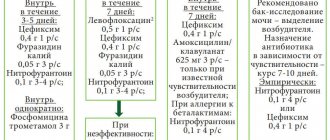
Antimicrobial therapy should be long-term (6–12 months) and based on urine culture results. The most convenient for children are oral medications. For hospital treatment, intramuscular or intravenous forms are used. The following drugs have been used to eradicate the pathogen:
- β-lactam semisynthetic penicillins:
– augmentin, 25–50 mg/kg/day, orally for 7–10 days;– amoxiclav, 20–40 mg/kg/day, orally for 7–10 days;
- 2nd generation cephalosporins:
– cefuroxime (zinnate), 20–40 mg/kg/day (in two doses) for 7–10 days;– cefaclor (ceclor), 20–40 mg/kg/day (in three doses) for 7–10 days;
- 3rd generation cephalosporins:
– cefixime (Suprax), 8 mg/kg/day (in 1 or 2 doses) for 7–10 days;– ceftibuten (cedex), 7–14 mg/kg/day (in 1 or 2 doses) for 7–10 days;
- fosfomycin (monural), 1.0–3.0 g/day;
After using bactericidal drugs (antibiotics) for a long course, uroseptic therapy is prescribed:
- nitrofuran derivatives: nitrofurantoin, 5–7 mg/kg/day, orally, for 3–4 weeks;
- quinolone derivatives (non-fluorinated):
– nalidixic acid – 60 mg/kg/day, orally, for 3–4 weeks;– pipemidic acid (pimidel, palin) - 200–400 mg/day, orally, for 3–4 weeks;
– nitroxoline (5-NOK) - 10 mg/kg/day, orally, for 3–4 weeks.
In order to relieve the allergic component, desensitizing agents are prescribed:
- tavegil 2 mg/day, orally, for 1–2 weeks;
- Claritin 5–10 mg/day, orally, for 1–2 weeks;
- fenistil 2–4 mg/day, orally, for 1–2 weeks.
An important role is played by the use of drugs that improve intracellular metabolism. For antioxidant purposes the following are prescribed:
- tocopherol acetate, 1–2 mg/kg/day, orally, for 3–4 weeks;
- B-carotene - at the rate of 1 drop per 1 year of life per day, orally, for 3-4 weeks;
To correct mitochondrial deficiency, the following are used:
- succinic acid 25 mg/day, orally, for 3–4 weeks;
- pyridoxine 2 mg/kg/day, orally, for 3–4 weeks.
An obligatory component of conservative therapy is the use of herbal remedies: leaves and fruits of lingonberries, cranberries, currants, oak bark, St. John's wort, nettle, chamomile, blueberries, coltsfoot, etc.
To increase the effectiveness of treatment of cystitis in older children, local therapy is used - intravesical instillations, which should be treated with caution in patients with high degrees of VUR. It is important to remember that the volume of solutions should not exceed 20–50 ml. Solutions used: protargol; solcoseryl; hydrocortisone; chlorhexidine; furacillin.
The course of treatment is designed for 5–10 instillations; for bullous cystitis, 2–3 courses are repeated. It is effective to supplement local therapy with physiotherapeutic treatment.
A special place in the treatment of urinary tract infections in children should be given to normalizing intestinal function. Disruption of the normal rhythm of colon emptying leads to compression of the lower third of the ureter, impaired vascularization, congestion in the pelvic area, and infection of the urinary tract (lymphogenous tract).
NDMP is observed in patients with myelodysplasia and its various manifestations: meningomyelocele, rachischisis, Spina bifida (incomplete closure of the spinal canal), etc. The treatment of NDMP is an important part of complex therapy. Elimination of functional disorders of the urinary tract is a complex task and requires a long time.
For hyporeflex detrusor it is recommended:
- mode of forced urination (after 2–3 hours);
- baths with sea salt;
- glycine 10 mg/kg/day, orally, for 3–4 weeks;
- physiotherapy: electrophoresis with proserin, calcium chloride, ultrasound on the bladder area, electrical stimulation;
- intermittent sterile catheterization of the bladder.
For detrusor overactivity, the following are prescribed:
- tolterodine (detrusitol) 2 mg/day, orally, for 3–4 weeks;
- oxybutynin hydrochloride (driptan) 10 mg/day, orally, for 3–4 weeks;
- trospium chloride (spazmex) 5 mg/day, orally, for 3–4 weeks;
- tamsulosin (omnic), doxazosin (cardura) 1 mg/day, orally, for 3–4 weeks;
- nicotinol-gamma-aminobutyric acid (picamilon) 5 mg/kg/day, orally, for 3–4 weeks;
- imipramine (melipramine) 25 mg/day, orally, for 3–4 weeks;
- desmopressin (minirin) 0.2 mg/day, orally, for 3–4 weeks;
- biofeedback;
- physiotherapy: electrophoresis with atropine, papaverine, ultrasound on the bladder area, electrical stimulation of the bladder using a relaxing technique, magnetic therapy.
Physiotherapeutic treatment is auxiliary in nature, but significantly increases the effectiveness of therapy. This method is used both for LUTD and inflammatory diseases of the urinary tract.
A special place in the treatment of PMR is given to endoscopic methods. Thus, according to a survey conducted by Italian urologists, 80% of parents choose endoscopic treatment as an alternative to open surgery and long-term drug therapy [7].
The priority in the medical use of Teflon paste belongs to the otolaryngologist Arnold, who used it to correct the glottis (1962). Teflon was introduced into urological practice in 1974, when V. Politano performed a paraurethral injection for urinary incontinence. For the first time, E. Matouschek announced a minimally invasive endoscopic method for correcting VUR. In 1981, with positive results, he insufflated Teflon paste into an 8-year-old child at the mouth of a refluxing ureter [8]. Subsequently, O. Donnel together with P. Puri (1984) described a method of endoscopic treatment of VUR [9]. Over 25 years, a large number of implantable materials have been tested, from Teflon to autogenous cell cultures [8–16]. The most complete classification of injectable materials is as follows [17]:
- auto- and allogeneic: blood, fat, chondrocytes, detrusor cells, human collagen;
- xenogeneic and synthetic: Teflon, silicone, dextranomer/hyaluronic acid, calcium hydroxyapatite, bioglass fiber, polyvinyl alcohol foam, hydroxyethyl methacrylate, bovine collagen, durasphere B.
The first experience with the use of antireflux implants alarmed specialists due to the possibility of developing undesirable side effects: the occurrence of necrosis at the injection site, malignancy, migration of material with the formation of granulomas in regional lymph nodes and/or parenchymal organs [18–20]. Modern experimental and clinical studies have proven the inertness, hypoallergenicity, and safety of currently used biomaterials [12, 13]. Positive results of endoscopic treatment of VUR, according to recent studies (Chertin, 2003; Kirsch, 2004) reach 70–90%.
Indications for the endoscopic method of correction of VUR are the ineffectiveness of conservative therapy within a period of 6 to 12 months. The absence of an acute phase of bladder inflammation is a prerequisite for endocollagenoplasty.
The endoscopic method of correcting VUR, due to its ease of implementation and fairly high efficiency, is firmly included in the algorithm of treatment tactics. Undoubtedly, an important role should be given to the correct choice of indications for this method. Collagenization of the ureteric orifice is justified if its structure is normal, i.e., there is no gaping of the orifice and lateral dystopia. In cases of IV and V degrees of VUR and a decrease in renal secretory function >50%, open reimplantation of the ureter is indicated.
The endoscopic treatment method, aimed at lengthening the intramural portion of the ureter and reducing the diameter of the orifice, is performed according to the following technique. Cystoscopy is performed, during which the clinical and anatomical picture of the bladder is determined: the condition of the bladder triangle, the shape and location of the ureteral orifices, the presence of paraurethral diverticula and ureterocele, and inflammatory changes. A long injector (diameter 5Ch) is passed through the working channel of the cystoscope tube, attached to a syringe with collagen. Collagen is presented as a substance in the form of a gel. The standard volume of collagen in a syringe is 2 ml. The injector needle (length 6 mm) is inserted under the mouth of the ureter - at 6 o'clock on the conventional dial, to the entire depth. To prevent complications before collagenoplasty, catheterization of the orifice with a 5Ch ureteral catheter is possible. When pressure is applied to the piston of a syringe with collagen, a roller gradually forms in the area where the needle is inserted. Depending on the degree of gaping of the mouth and the length of the submucosal section of the ureter, 1 to 2 ml of the substance is injected. In this case, the mouth of the ureter acquires a point or slit-like shape, after which the instrument is removed. The bladder is drained for 24 hours with a double-lumen urethral Foley catheter 8–14Ch, the balloon of which is filled with 5 ml.
The most effective method for correcting VUR remains surgical – 93–97% of cases [21]. More than 80 methods of open surgical treatment of VUR are known - these are various modifications of ureterocystoanastomosis. The principle mechanism of all types of open procedures is the lengthening of the intramural part of the ureter in order to create a valve mechanism capable of passing urine in one direction from the ureter to the bladder. The tunnel anastomosis technique is recognized as the most successful, due to the ability of the tunnel to withstand high hydrostatic pressure inside the bladder (both during a full bladder and during urination), preventing the reflux of urine into the ureter.
Indications for surgical treatment are: refluxing megaureter (with PMR of IV, V degrees), recurrent nature of PMR at lower degrees and the absence of a positive effect from conservative or endoscopic treatment, a combination of reflux with an obstructive component in the PMS zone.
In the postoperative period, all patients must undergo prophylactic antibiotic therapy for 3–4 days, followed by a transition to uroseptic therapy for 2–3 weeks.
Publications concerning the first experience of using the tunnel technique of ureterocystoanastomosis belong to D. Williams and J. Hutch and they appeared in the 60s of the last century [3]. According to various sources, from 80 to 120 methods of open surgical treatment of VUR are known - these are various modifications of ureterocystoanastomosis. Some are performed with opening of the bladder (Cohen, Politano-Leadbetter, etc. operations) [22], others without cystotomy (Leach-Gregoire [23, 24], Barry [22] operations). At any age, these operations are performed using endotracheal anesthesia. Complications of open operations in most cases are common - bleeding, development of anastomotic stricture, impaired urine evacuation at the level of the vesicoureteral junction as a result of angulation and, as a consequence, the development of retention of the upper urinary tract, relapse of VUR, postoperative cystitis and pyelonephritis [25].
In Russia, the Cohen and Politano-Leadbetter operations are most often performed. Cohen's ureterocystoanastomosis is performed through an incision in the anterior wall of the bladder and is based on the principle of lengthening the intravesical part of the ureter by reimplanting it into the newly formed submucosal tunnel. Specific complications of this method are bleeding from the vesical triangle (Lieto) and the juxtavesical ureter, and the development of postoperative cystitis. Postoperative bleeding from Lieto's triangle is associated with the formation of a submucosal tunnel in the most blood-supplied area of the bladder, which is due to anatomical features. Postoperative bleeding from the juxtavesical ureter occurs due to rupture of the regional arterial and venous plexuses during blind traction for passage through the submucosal tunnel. Both types of bleeding require repeated revision of the surgical wound, hemostasis and worsen the result of reconstructive plastic surgery. Due to the transvesical approach, the features and weaknesses of Cohen's ureterocystoanastomosis are the intersection of all layers of the bladder wall with the development of a scar that prevents normal contractility of the bladder; the impossibility of straightening the kinks of the dilated ureter and performing its modeling before reimplantation, the need for which arises in grades IV and V VUR (grades IV and V account for up to 60% of all surgical interventions).
The principle of ureterocystoanastomosis according to Politano-Leadbetter is the creation of a submucosal tunnel of the bladder. A feature of the technique is a wide opening of the bladder and dissection of the mucous membrane of the bladder in three places to create a tunnel, while the ureter is cut off from the outside of the bladder, since this method involves resection of the dilated ureter. Specific complications of the Politano-Leadbetter operation are the development of a scar at the site of the opening of the bladder; development of angulation of the prevesical ureter due to the use of anastomosing technique and the formation of strictures of the vesicoureteral anastomosis that are not amenable to endoscopic correction. A characteristic radiological symptom of ureteral angulation is its transformation in the form of a fish hook. In practice, this significantly reduces the possibility of catheterizing the kidney if necessary (for example, with urolithiasis). The use of such a traumatic method of ureterocystoanastomosis in the absence of dilation of the ureter is limited or unjustified.
The extravesical method of ureterocystoanastomosis is the most effective surgical intervention in children with VUR (98%). The tasks of ureterocystoanastomosis include the creation of a reliable valve mechanism of the PMS, the formation of an adequate lumen of the ureter that does not interfere with the free passage of urine. The extravesical technique of ureterocystoanastomosis fully meets the requirements. The use of the extravesical technique allows one to avoid opening the bladder (wide dissection of the detrusor) and at the same time makes it possible to form a submucosal tunnel on any part of the bladder wall, choosing an avascular zone. The length of the tunnel can also be chosen arbitrarily by the surgeon.
The emergence of new effective drugs for the pharmacological conservative treatment of VUR in children, on the one hand, and the introduction into practical medicine of new surgical treatment methods, including laparoscopic surgery and robotics, on the other, will certainly change the meaning of conventional open methods for correcting VUR in the future. But today, in the treatment strategy for VUR, open surgical interventions play a key role, as they make it possible to eliminate the most complex forms of VUR.
In conclusion, I would like to emphasize that the choice of a method for correcting VUR should be strictly individual and based on the doctor’s objective analysis of examination data and an assessment of the possibility of using all available methods for eliminating VUR in this particular patient.
Literature
- Yatsyk P.K., Zvara V. Vesicoureteral reflux in children. M., 1990. pp. 41–44.
- Kokolina V.F., Rumyantseva A.G. Practical guide to childhood diseases. Nephrology of childhood. M.: Medpraktika-M, 2005. T. VI. pp. 234, 240, 252.
- Lopatkin N. A., Pugachev A. G. Vesicoureteral reflux. M., 1990. S. 5, 119.
- Campbell M. Clinical Pediatric Urology. 1951; 164.
- Isakov Yu. F. Surgical diseases in children. M., 1998. P. 327.
- Vishnevsky E. L. Overactive bladder in children / Current issues in the diagnosis and treatment of urological diseases in adults and children. Tyumen, 2005. P. 324.
- Capozza N., Lais A., Matarazzo E. et al. Treatment of vesico-ureteric reflux: a new algorithm based on parental preference//BJU international. 2003; 92:285–288.
- Matouschek E. Treatment of vesicoureteral reflux by transurethral teflon injection//Urologe A. 1981; 20: 263–264.
- Puri P., O'Donnell B. Correction of experimentally produced vesicoureteric reflux in the piglet by intravesical injection of Teflon//Br. Med. J. 1984; 289:5–7.
- Nemenova A. A., Chepurov A. G. Endoscopic correction of vesicoureteral reflux by injection of Teflon paste // Urology and Nephrology. 1993. No. 2. P. 7–10.
- Puri P., Chertin B., Dass L. Treatment of vesicoureteral reflux by endoscopic injection of dextranomer/hyaluronic acid copolymer: preliminary results//The Journal of Urology. 2003; 170:1541–1544.
- McPherson JM, Wallace DG, Piez KA Development and biochemical characterization of injectable collagen//J. Dermatol. Surg. Oncol. 1988; 14(1):13.
- Alkan M., Talim B., Ciftci AO, Senocak ME, Caglar M., Buyukpamukcu N. Histological response to injected gluteraldehyde cross-linked bovine collagen based implant in a rat model//BMC Urology. 2006; 6:3.
- Paradysz A., Fryczkowski M., Krauze-Balwinska Z., Gajewski D. Comparison of effectiveness of endoscopic injection of autologous blood and conservative therapy in the treatment of bilateral primary vesicoureteral reflux//Wiad. Lek. 2002; 55 (7–8): 404–410.
- Kireeva N. B., Khafizova L. A., Parshikov V. V., Zaugarov M. Yu., Aleynik D. Ya. Endoscopic correction of vesicoureteral reflux in children using auto- or allofibroblasts // Nizhny Novgorod Medical Journal. 2003. No. 3–4. pp. 8–12.
- Babanin I. L., Kazanskaya I. V., Konoplev V. D. Efficacy of endoscopic treatment of vesicoureteral reflux in children using bioimplants: materials of the X Russian Congress of Urologists. M., 2002. pp. 698–699.
- Ander AH Endoscopic treatment of vesicoureteral reflux. Abstract Book from 8th Mediterranean video-endoscopic urology and European society of urological technology. 2006; 13–14.
- Larsson E., Stenberg L. et al. Injectable dextranomer-based implant: histopathology, volume changes and DNA-analysis//Scandinavian Journal of urology and Nephrology. 1999; 33 (6) 355–361.
- Mittleman RE, Marraccini JV Pulmonary Teflon granulomas following periurethral Teflon injection for urinary incontinence//Arch. Pathol. Lab. Med. 1983, 107: 611–612.
- Aaronson IA, Rames RA, Greene WB, Walsh LG, Hasal UA, Garen PD Endoscopic treatment of reflux: migration of Teflon to the lungs and brain//Eur. Urol. 1993, 23: 394–399.
- Lopatkin N. A., Pugachev A. G., Apolikhin O. I. //Urology. M., 2002. P. 118.
- Hinman F. Operative urology. M., 2001. pp. 811–819.
- Lich R., Jr., Howerton LW, Davis LA Recurrent urosepsis in children//J Urol. 1961; 86:554.
- Gregoir W. Le traitement chirurgical du reflux vesico-ureteral congenital // Acta. Chir. Belg. 1964; 63:432.
- Polyakov N.V. Evaluation of the effectiveness of reconstructive plastic surgeries on the vesicoureteral segment in children: dis. ...cand. honey. Sci. M., 2003. pp. 111–119.
A. Yu. Pavlov, Doctor of Medical Sciences S. A. Maslov N. V. Polyakov , Candidate of Medical Sciences A. A. Lisenok , Candidate of Medical Sciences G. V. Simonyan Research Institute of Urology, Moscow
How is the catheterization procedure performed?
The child is placed on a medical couch and asked to bend his knees and spread them apart. The external genitalia and urethra are treated twice with an antiseptic solution.
In boys, a sterile napkin is wrapped around the head of the penis 2 cm below the urethra. For girls, the labia are covered with sterile napkins.
One end of the sterile catheter is taken with the thumb and index finger of the right hand wearing sterile gloves. The second end pointing upward is held with the little finger.
With the left hand in a sterile glove, the tissues in the urethral area are separated and a catheter is inserted into it. The second end is lowered into the urinal.
After urine has almost ceased to be excreted, medications are administered if necessary. If the catheter is temporary, apply gentle pressure to the bladder and slowly remove the tube. If the catheter is permanent, it is fixed with an adhesive tape.
How does a Foley catheter work?
Let's briefly look at what this medical catheter looks like and its principle of operation. This is a long tube, at one end of which there is a smooth tip with two slits through which urine is drained from the bladder, and just below them a balloon is inflated, preventing the product from falling out of the body by blocking the neck of the bladder from the inside. At the other end of the tube there are:
- hole through which urine is drained;
- a separate hole through which the cylinder is filled;
- special anti-reflux valve.
Three-way catheters also have an additional hole through which medications can be administered.
These types of catheters are used for those patients who require catheterization within a week to a month (or longer). They are very effective against urinary retention. Urine begins to flow immediately after installing the device. The basic principle of operation is the passive outflow of urine from the bladder through the holes into the tube and urinal.
Why you should contact us
We have been working since 2002 - during this time we have earned the trust of patients. We provide written guarantees of the quality of medical services. We provide expert pediatric urology - young patients are treated by highly qualified doctors, candidates and doctors of medical sciences, professors and academicians. If necessary, we quickly organize consultations - we employ 850 multidisciplinary specialists.
We have our own laboratory, so we do tests quickly on site. We use catheters from the world's leading manufacturers, so we carry out catheterization for children painlessly, delicately, safely, quickly and accurately.
Convenient reception schedule – we work every day, including weekends and holidays. It’s convenient to get to us - our 4 clinics are located in different areas of St. Petersburg.
To make high-quality pediatric urology more accessible, there is a discount system for patients and discount promotions are held. For more information, call us at the number provided 24 hours a day.