Story
P. p. is the most promising in the problem of organ transplantation. The constant increase in the number of these operations (according to the International Registration Center for Organ Transplantation, by 1969, 2,347 kidney transplants were performed, by 1977 - more than 30,000) and the expansion of the network of kidney centers (see) throughout the world (by 1980 .— St. 500) indicate the effectiveness of P. p.
The scientific study of the problem of PP began with the experimental work of A. Carrel (1902), E. Ullmann (1902), A. Decastello (1902), and Unger (1910). In experiments on various animals, a technique for restoring the main blood flow during auto- and allotransplantation of the kidney was developed, the possibility of using hypothermic perfusion to significantly reduce the development of ischemic damage in the kidney was revealed, and it was shown that renal function was restored after ischemia within 1 hour. The experiments also helped to identify certain signs of transplant rejection.
In 1902, the Austrian surgeon Ullmann performed the first xenotransplantation of a kidney, transplanting a pig kidney into the arm of a patient with uremia. Similar operations were attempted by other scientists, but without success.
A new direction in scientific research was laid down by the Soviet scientist Yu. Yu. Vorony. In 1934, he reported that he had performed a kidney transplant (taken from a corpse) to a woman who was dying of acute renal failure, which developed as a result of sublimate poisoning. The donor kidney was taken from the corpse of a man who died as a result of a traumatic brain injury. Despite the unfavorable outcome (the patient died 2 days later in the absence of function of the transplanted kidney), this operation should be considered a new qualitative stage in the history of transplantology (see). Later, a kidney transplant taken from a corpse was carried out by a number of foreign surgeons - JIoypep (Lawrer) et al. (1950), Ch. Dubost, M. Servelle et al. (1951), R. Kiiss et al. (1951), etc., but due to the lack of immunosuppressive therapy, they were unable to achieve long-term functioning of the transplanted organ. The results of kidney transplants taken from close relatives were more successful. Thus, Hume (D. M. Hume, 1953) observed a patient with a five-month period of functioning of a kidney transplanted from an identical twin, L. Michon et al. (1953)—for several weeks.
Achievements in immunology (see) and immunotherapy (see), the introduction into the wedge, the practice of immunosuppressive substances (see) have made it possible to significantly improve the results even when transplanting a kidney taken from a corpse. That is why in the 60s. In many countries of the world, the rapid spread of P. p. begins.
In the USSR, the first successful kidney transplantation from a living donor (from the mother of a daughter) was performed in 1965 by B.V. Petrovsky. A few months later, he also performed P. p. using a graft from a corpse. By 1980, St. 2000 operations, not only in leading centers (All-Union Scientific Center of Surgery of the USSR Academy of Medical Sciences, Institute of Transplantology and Artificial Organs of the USSR Academy of Medical Sciences, urological clinic of the 2nd MMI), but also in kidney centers of other large cities - Leningrad, Kiev, Vilnius, Riga, Minsk, Alma-Ata, Kemerovo, Tashkent, etc.
The network of transplantation centers continues to expand, and kidney donation and its organization are also being improved, immunol is being studied. compatibility of donor and recipient, new means of immunosuppression, medical examination and rehabilitation of patients after surgery are being developed.
The operation is performed on patients aged 16-50 years, but there are many reports of P. p. in children and people over 55 years of age.
Alarming Consequence
Full speed ahead: overweight people accused of spreading coronavirus
These patients may be exhaling more of the virus, according to researchers.
Scientists at Yale University School of Medicine conducted a study involving 1.6 thousand patients with acute kidney injury. Experts monitored people who were diagnosed with pathology while in hospital with coronavirus, as well as those who did not survive the infection. According to American scientists, 24–57% of hospitalizations with COVID-19 and from 61% to 78% of hospitalizations in intensive care units are accompanied by kidney damage. Doctors monitored the condition of patients 21 days after discharge and found that those who had suffered coronavirus infection did not recover their original kidney function. Moreover, they were more likely to require dialysis than non-COVID-19 patients with acute kidney inflammation. Those who had the infection also had a higher risk of developing chronic renal failure (CRF), the study said.
“These data suggest that patients recovering from acute kidney injury associated with COVID-19 require monitoring of their function after hospital discharge,” the article said.
Berries later
Photo: RIA Novosti/Valery Melnikov
We got to the bottom of the mice: where they looked for the source of coronavirus
WHO experts did not find the causative agent of COVID-19 at the now world famous market in Wuhan
It is known that coronavirus infection can affect all vital organs and systems.
“The virus enters the epithelium, where it multiplies, and then enters the blood and attacks targets, which can be both the internal epithelium (gastrointestinal tract, lungs, genitourinary system) and red blood cells - erythrocytes,” a professor at the Department of Fundamental Medicine told Izvestia FEFU School of Biomedicine Galina Reva. “Although most often we see pathology of the respiratory system, the virus only needs the epithelial cell of the lungs for reproduction.
Currently, doctors have extremely limited scientific data on the effect of the virus on kidney tissue, noted clinical diagnostic urologist Artur Tedeev in an interview with Izvestia. So far, few autopsy studies have been published, since due to the high risk of the spread of the disease throughout the world, restrictive measures remain on autopsies of the bodies of those who died from COVID-19, he pointed out.
Berries later
Technical room at the Hemodialysis Center in Moscow
Photo: RIA Novosti/Valery Melnikov
— Available data indicate a direct effect of the virus on kidney tissue. It can cause the development of acute renal failure, which is microscopically defined as severe acute tubular necrosis. Most often, this complication develops in people with cardiovascular pathologies (hypertension, coronary heart disease) and diabetes,” the specialist said.
Sources for obtaining a kidney for transplantation
There are three sources of obtaining a kidney for transplantation: living donors, cadavers, and apes. The group of living donors includes relatives of the patient and unrelated volunteers. Kidneys are rarely taken from unrelated living donors due to the lack of genetic relatedness to the recipient.
Taking a kidney from living related donors (mother, father, sister, brother) has the advantage that tissue compatibility tests can be performed and the most suitable donor can be selected. The operation is performed as planned, and therefore with the most rational preparation. Taking a kidney from a living related donor is not a completely safe procedure; mortality, according to J. Amburger (1965), is 0.05%.
The entire complex of immunol examinations aimed at identifying the degree of tissue compatibility (see Immunological incompatibility) must be carried out in the following sequence. First of all, compatibility is determined by erythrocyte antigens of the AB0 system and the Rh factor (see). Then it is determined whether there are lymphocytotoxic antibodies in the recipient's blood. It is necessary to carry out the so-called cross-test and determine the antigenic compatibility of leukocytes according to the HLA system (see Blood groups).
Regarding the choice of a donor for a kidney transplant from a corpse, despite the accumulated experience, there are different opinions, in particular about the age of the donor.
Contraindications to the use of a kidney from a corpse: malignant tumors in donors, with the exception of primary brain tumors that do not metastasize; malignant hypertension; urinary tract infection; septicemia; pneumonia; hepatitis and other inf. liver diseases; history of kidney disease; prolonged agony with prolonged hypotension. Of great importance in assessing the possibility of using a donor is the determination of kidney function before his death, based on measuring diuresis, determining the density of urine, the content of urea and creatinine in the blood.
To preserve kidneys removed from a corpse, a non-irfusion hypothermic method is often used using various solutions (see Preservation of organs and tissues). In the USSR, solutions developed at the Institute of Organ and Tissue Transplantation and the All-Union Scientific Center for Surgery are widely used. The period of relatively safe kidney preservation using the non-perfusion method is 24 hours.
The use of a kidney from an ape (xenotransplantation) is practically abandoned, because severe tissue incompatibility leads to rapid death of the graft. The longest period of xenograft functioning does not exceed 9 months.
Damaging mechanism
Rare sense of smell: COVID loss of smell boosts immune response
There are many cells in the nose that the coronavirus binds to, which causes activation of lymphocytes and stronger production of antibodies
Previously, another group of American doctors led by Yale University professor Choukri Ben-Mamoun proved that coronavirus infection often leads to the development of albuminuria (a sign of kidney dysfunction in which protein is released in the urine) and other kidney problems. Using a special test, scientists found fragments of SARS-CoV-2 proteins in the urine of a quarter of patients at two American clinics being treated for COVID-19. The presence of the virus in the kidneys of patients was accompanied by an abnormally high concentration of albumin in the urine, which indicates serious damage to the kidneys, according to the results of the study, published in the electronic library medRxiv.
“There is no specific treatment for acute renal failure yet, so patients with this pathology need to undergo therapy that supports kidney function,” said Artur Tedeev. — In severe cases of the disease, it is important first of all to control the level of plasma creatine, the volume of urine excreted, proteinuria, microhematuria, as well as water balance in order to avoid pulmonary edema, overload of the right ventricle and acute damage to the kidney tubules.
Indications and contraindications
The main indication for P. p. is the terminal stage of chronic renal failure. More often P. item is made to persons suffering from chronic. glomerulonephritis, hron, pyelonephritis, polycystic kidney disease, as well as patients who have lost their only kidney as a result of injury or surgery.
Contraindications to P. p. have not been definitively determined. As a rule, P. p. is not indicated for mentally handicapped people, patients with systemic vasculitis or diffuse connective tissue diseases, tuberculosis, malignant neoplasms, diabetes mellitus, severe cachexia, malignant hypertension, amyloidosis, uncorrectable urinary tract defects, and severe atherosclerosis. If renal failure is accompanied by chronic disease, hepatitis, often of viral etiology, transplantation becomes dangerous due to the need to further use the immunosuppressant imuran (azathioprine), which has a hepatotoxic effect. Pathology went.-intestinal. tract, often accompanying hron, uremia - uremic gastritis, even erosive ulcerative enterocolitis - are not an absolute contraindication to kidney transplantation, since adequate hemodialysis, antacids, a gentle diet can completely eliminate these conditions. With concomitant gastric and duodenal ulcers, there is a high risk of its exacerbation in the post-transplant period, especially against the background of immunosuppressive therapy. In cases of unsuccessful conservative therapy, especially with hypersecretion of gastric juice, gastrectomy, vagotomy (stem or selective) and pyloroplasty are considered appropriate in preparation for transplantation in such patients; only after this is P. p. possible.
Preparing for surgery
Preparation for surgery is carried out mainly in the following areas: elimination of uremic intoxication and its complications, correction of water-electrolyte metabolism and acid-base balance; sanitization of foci of infection and elimination of generalized infection that has arisen; fight against anemia, dysproteinemia; elimination of hypertension, especially those with a malignant course, stabilization of cardiovascular system indicators.
Preparation for surgery is provided by a set of measures: hemodialysis, drug and transfusion therapy, and sometimes surgical aids. In this complex, the leading place is occupied by hemodialysis (see), particularly high demands are placed on it in transplantation centers.
Methodology
Kidney transplantation is usually performed heterotopically, into the left or right iliac fossa. Orthotopic P. and. practically not used, because it is not only technically more complex, but is also accompanied by a large number of various complications.
Due to anatomical features, it is better to transplant the left kidney into the right iliac fossa, and the right kidney, on the contrary, into the left. If necessary, you can deviate from this rule.
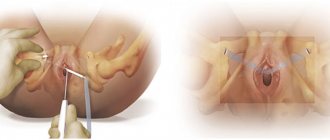
Schematic representation of kidney transplant options. A. The most common option: 1 - kidney graft, 2 - anastomosis of the renal artery with the internal iliac artery, 3 - anastomosis of the renal vein with the common iliac vein, 4 - anastomosis of the ureter (located under the serous membrane) with the bladder; at the top left, a black line shows a section of the soft tissues of the right iliac region. B. Option for anastomosing the renal artery using a section of the wall of the abdominal aorta during kidney transplantation: a - taking a graft from a donor with a section of the aortic wall; 1 — kidney graft, 2 — renal artery, 3 — renal vein, 4 — excision of a section of the wall of the abdominal aorta, 5 — ureter; b — an anastomosis of the renal artery was performed (using the wall of the abdominal aorta) with the internal iliac artery; 1 — kidney transplant, 2 — anastomosis of the renal and internal iliac arteries, 3 — renal vein, 4 — abdominal aorta, 5 — ureter, 6 — internal iliac vein.
The operation is usually performed under endotracheal anesthesia. Access - an oblique incision in the right (Fig. A) or left iliac region parallel to the inguinal fold. The iliac fossa is opened extraperitoneally, the peritoneum is peeled off over a wide area, and a kidney is placed in the resulting space. First, the vessels of the kidney transplant are connected to the internal iliac artery and the common iliac vein (Fig., B) end to end or end to side. Sometimes, to prevent stenosis in the area of arterial anastomosis, a graft is taken from a section of the aortic wall and used to connect to the internal iliac artery (Fig. B a and b).
The critical step in P. p. is the restoration of continuity of the urinary tract. For this purpose, ureterocystostomy is most often used. Interureteral or ureteropelvic anastomoses are performed much less frequently, since in this case, suture failure often occurs and urinary fistulas form. There are two methods for creating ureterocystoanastomosis - intravesical and extravesical; The most widespread method is the extravesical method of creating ureterocystoanastomosis.
With P. from a living related donor, surgical intervention has a number of features. Taking the graft from the donor and isolating the iliac vessels from the recipient are performed in parallel by two teams of surgeons. Nephrectomy (cm), both unilateral and bilateral, is carried out from an oblique retroperitoneal approach, which is carried out along and slightly below the XII rib. Anteriorly, the incision reaches the rectus abdominis muscle, and then continues down the pararectal line for 4-6 cm. The kidney is carefully freed from perinephric tissue, and then the renal artery and vein are isolated; the artery is isolated to the aorta, and the vein to the point where it flows into the inferior vena cava (this is especially important if you take the right kidney, which has a relatively short vein, the length of which is 4-5 cm). Then the ureter is freed from the surrounding tissues for 15-20 cm and crossed, its distal end is bandaged. The renal artery and vein are ligated and divided sequentially. After removing the kidney from the wound, it is perfused with a preservative solution cooled to 4°C and delivered for transplantation to the recipient. The further stages of transplantation do not differ from P. p. from a corpse. When the kidney is included in the bloodstream, the clamps are sequentially removed from the venous and arterial lines. Within a few minutes the kidney acquires a pink color and normal turgor. With good function and preservation, urine begins to be released from the ureter.
The function and condition of the graft can be judged with great certainty by intraoperative determination of volumetric blood flow in the renal artery and intrarenal vascular resistance. The amount of volumetric blood flow is determined using an electromagnetic flowmeter.
Postoperative period
Severe uremic lesions of internal organs, observed in patients in the terminal stage of chronic disease, renal failure, transplantation of a foreign organ into the recipient's body and immunosuppressive therapy, which is itself toxic, require the doctor to carefully monitor the condition of all organs and systems.
One of the basic principles of patient management in the post-transplant period is careful adherence to the rules of asepsis (see) and antisepsis (see), especially during dressing changes, since corticosteroids, being the main component of immunosuppressive therapy, suppress not only transplant immunity (see Transplant immunity) , but also inhibit the patient’s body’s resistance to infection. Monitoring the patency of the catheter inserted into the bladder is important, since its blockage leads to overflow of the bladder, failure of the ureterovesical anastomosis and subsequent formation of a urinary fistula.
Management of the patient in the postoperative period, which occurs in the presence of a well-functioning transplant, and in the postoperative period against the background of oliguria or anuria, is different.
When assessing diuresis after transplantation (see Diuresis), the amount of urine excreted by the patient before surgery should be taken into account. If the amount of diuresis after surgery remains the same, this should alert us to the development of anuria due to insufficient graft function.
Almost all living related donor transplants and a certain percentage of cadaveric kidneys begin to produce urine immediately after they are introduced into the bloodstream, and within the first 24 hours. diuresis is several liters. Less commonly observed is severe polyuria (see), in which diuresis reaches 10 liters or more. Such massive polyuria requires hourly monitoring of the amount of urine excreted and the concentration of potassium and sodium in it, adequate replenishment of fluid loss and the prevention of dangerous electrolyte imbalances.
Prevention of inf. complications are also very significant in the management of the post-transplant period. Broad-spectrum antibiotics must be prescribed in combination with antifungal drugs. During the first 2-3 weeks. UV irradiation of the wound area is recommended. To prevent pneumonia, breathing exercises are performed, expectorants and mustard plasters are prescribed.
ECG monitoring is necessary so as not to miss indications for the use of cardiotonic drugs, as well as correction of hypertension, which is often observed in such a group of patients and negatively affects cardiac activity and graft function.
In patients with good early function of the transplanted kidney, from the first hours of the postoperative period, an independent decrease in the concentration of nitrogenous wastes in the blood begins, which makes it possible not to resort to hemodialysis.
In 20-30% of patients after cadaveric kidney transplantation, oliguria (see) or anuria (see), which is a consequence of necrobiotic changes in the tubular epithelium of the graft of anoxic origin, is observed. The cause of oliguria or anuria may be acute ischemic tubular necrosis, hyperacute transplant rejection, thrombosis of vascular anastomoses, ureteral obstruction, urinary leakage with compression of the transplanted kidney.
Preservation of the viability of the transplanted organ during these complications depends on how quickly and in what sequence diagnostic measures are performed, which are advisable to carry out in the following order: 1) make sure that the volume of circulating blood and the degree of hydration are adequate; 2) exclude circulatory disorders in the graft; 3) establish the integrity of the urinary tract and its patency; 4) exclude a crisis of rejection; 5) as a last resort, perform exploratory intervention to exclude anuria of mechanical origin (ureteral obstruction, thrombosis of the vascular anastomosis).
If mechanical causes of anuria, a rejection crisis are excluded, and it is established that anuria is a consequence of ischemic necrosis of the tubular epithelium, it is necessary to resolve the issue of the reversibility of this process or the indications for transplant removal. Watchful waiting is rational only in the absence of a wedge, signs of toxic effects from the transplanted kidney.
Lack of graft function and absorption of metabolic products from the surgical wound lead to increased acidosis (see) and shifts in electrolyte balance - hyperkalemia (see), increased intoxication (see). Hemodialysis helps to significantly reduce the concentration of nitrogenous wastes and intoxication, as well as correct disturbances in water-electrolyte balance and acid-base balance. Indications for hemodialysis are basically the same as before transplantation. Due to the risk of bleeding from the wound, regional heparinization (that part of the patient’s blood that is in the device) is mandatory. The amount of fluid administered to patients with oliguria or anuria should be limited; for anuria, the amount of fluid administered intravenously and orally should not exceed 10 ml per 1 kg of the patient’s body weight.
All patients in the early postoperative period must undergo treatment. massage and exercise therapy starting from the second day after surgery.
One of the main tasks of managing patients after kidney transplantation is immunosuppressive treatment (see Immunotherapy), which is the basis for the successful outcome of kidney allotransplantation. For this purpose, corticosteroids (prednisolone, methylprednisolone), cytostatics (imuran, cyclophosphamide, bat-riden, actinomycin D), antilymphocyte or antithymocyte globulin (ALG, ATG), and X-ray irradiation of the transplanted kidney area are used. On the first day after surgery, prednisolone is prescribed at a dose of 1-2 mg per 1 kg of body weight, and imuran - 2-4 mg per 1 kg of body weight. Subsequently, the dose of these drugs varies depending on the nature of the post-transplant period; by the end of the first month, the dose of prednisolone in uncomplicated cases is reduced to 0.25-0.5 mg per 1 kg of weight.
Timely diagnosis of a rejection crisis is one of the main difficulties in managing patients after transplantation. In the early postoperative period, when there is still no stable balance between the transplanted organ and the recipient’s body, the risk of developing this complication is especially great. Acute rejection develops predominantly in the early postoperative period, hron, rejection - in the long-term period. Rejection crises in the first 3 months. after transplantation occur in approximately 60% of patients. Diagnosis of rejection crises is based on the use of a whole complex of clinical, biochemical, instrumental and immunological tests. Such a variety of diagnostic methods indicates that none of them is absolutely reliable.
Signs of acute kidney transplant rejection
Clinical: increased body temperature with a peak in the morning and afternoon, persistent hypertension, decreased diuresis, pain on palpation of the transplant area, increased size of the transplanted kidney and its hardening.
Biochemical: increased concentration of creatinine in the blood, decreased clearance, increased level of lysozyme in the urine.
Instrumental: with ultrasonic echolocation of the graft, an increase in its size and an increase in the thickness of the cortical substance by 1/2-2 times is noted compared with the initial data; a radioisotope study of hippuran labeled 1311 shows deterioration in the secretory and excretory segments of the renogram, which indicates a decrease in the function of the proximal nephron.
Immunological: increased levels of lymphotoxins, absolute and relative numbers of T-lymphocytes and decreased titer of the spontaneous rosette formation inhibition reaction.
Morphological: in the graft there is swelling of the interstitium, infiltration of lymphoid cells, aggregation of blood cells in the capillaries and arterioles of the glomeruli.
With the development of a rejection crisis, immunosuppressive treatment is intensified. Increase the dose of prednisolone prescribed orally to 1-1.5 mg/kg, while methylprednisolone is administered intravenously at a dose of 500-1000 mg per day (3-5 times every other day). X-ray irradiation of the area of the transplanted kidney is carried out 2-4 times at 150 rad.
In the postoperative period, complications associated with taking immunosuppressants may develop. Imuran (azathioprine) can cause bone marrow suppression and toxic hepatitis. Corticosteroids can cause the formation of ulcers. tract, sometimes complicated by bleeding; contribute to the occurrence of steroid diabetes, pancreatitis, Cushing's syndrome, hypertension, aseptic necrosis of the epiphyses of bones, myocardial infarction.
Surgical complications after P. p. include bleeding from the area of vascular anastomoses, retroperitoneum, thrombosis of graft vessels, rupture of a transplanted kidney, and lymphorrhea.
Urologic complications occur on average in 8-10% of patients. Most often, they are caused by failure of the vesicoureteral anastomosis. The formation of urinary fistulas and leaks is extremely dangerous and requires immediate surgical intervention in most cases. Acute obstruction of the ureter of a transplanted kidney can be caused by hl. arr. obstruction by a blood clot or kinking of the ureter.
To the main information. complications include suppuration of the surgical wound, sepsis, pneumonia, and graft pyelonephritis. To combat them, antibacterial therapy is prescribed under the control of the nature of the microflora and determination of the spectrum of its sensitivity to antibiotics.
P.'s experience indicates that stable immunol, balance between the graft and the recipient's body is established within 3 months. after operation. Therefore, 4th month. can be considered the beginning of the long-term postoperative period. In an uncomplicated course, it is characterized by stable function of the transplanted kidney and normal homeostasis indicators. If there is no rejection crisis, immunosuppressive therapy is stable: the dose of prednisolone does not exceed 10-15 mg per day, the dose of imuran a is 150-200 mg per day.
Rejection crises in the long term develop less frequently and are not as pronounced. The remote period is more characterized by hron, rejection, which begins gradually and is characterized by a latent course. Proteinuria appears or intensifies against the background of stable function of the transplanted kidney, then a decrease in glomerular filtration occurs, followed by an increase in the concentration of nitrogenous wastes in the blood.
The most common complications of the long-term post-transplantation period include: pyelonephritis of the transplanted kidney, steroid osteodystrophy, myocardial infarction, diabetes mellitus, and imuran hepatitis. Less commonly, stenosis of the artery of the transplanted kidney develops, which is characterized by the presence of a systolic murmur over the area of the transplanted kidney, persistent hypertension and a progressive decrease in graft function. Successful reconstruction of a stenotic artery usually leads to a decrease in blood pressure to normal values and an improvement in the function of the transplanted kidney.
The imperfection of immunosuppressive therapy does not make it possible to obtain absolute tolerance of the recipient's body to the transplanted organ even in the long-term postoperative period, as a result of which the death of the transplant gradually occurs. Treatment The tactics in such cases boil down to removing the transplanted kidney and transferring the patient to chronic hemodialysis. After appropriate preparation, many patients undergo repeated P. p. (up to 3-4 times), which makes it possible to prolong their life for several more years.
Patients who have undergone a kidney transplant, their family and friends are afraid to even hear the word “rejection.” Most often, they are afraid that after everything they have been through, they will not be able to save the new kidney and will have to go back to dialysis.
It is true that a rejection episode can develop despite best efforts to prevent it. However, if rejection is detected in the earliest stages and treated quickly, in most cases the rejection process can be stopped and irreversible kidney damage can be avoided.
Understanding what rejection is and what to do to overcome it should help you and your loved ones get rid of anxiety. There is something sudden and inevitable in the word “rejection.” In fact, it does not happen so quickly and violently, and with good medical care it can generally be dealt with.
During an episode of rejection, the transplanted kidney (also called an allogeneic kidney transplant) becomes “sick” and cannot work as well as it should. This does not always mean that the kidney will completely stop functioning. Today, the medical team who performed your kidney transplant can do a lot to cure rejection and restore kidney health.
Nowadays, methods for selecting the appropriate organ before transplantation, surgical techniques, as well as medications that are used after surgery to prevent rejection have become much better and more sophisticated. Therefore, episodes of rejection or complete failure of a transplanted kidney are becoming less common. However, for some patients, one or more episodes of rejection are an expected part of the recovery process. Let’s try to figure out why your body may not “recognize” a transplanted kidney.
Why might rejection begin?
Your body has an amazing natural defense system. It's called the "immune system" and it protects you from anything foreign—things that aren't normally part of your body. Such “interveners” can be bacteria, viruses, as well as tissue from a transplanted kidney taken from another person. Even a common cold or flu activates the entire immune system!
Your immune system is like an “army” consisting of “soldiers”, which are special protective substances and cells. This is how they fight.
The frontline soldiers involved in the rejection process are blood cells called lymphocytes (a type of white blood cell). When the immune system encounters a foreign piece of tissue or cell (such a foreign substance is called an “antigen”), lymphocytes immediately spring into action to destroy the “strangers.”
Antigens are protein substances. Each of us has our own individual set of antigens. Another person's kidney has its own antigens, which may be different from yours. Therefore, your body perceives the transplanted kidney as something foreign. Sensing danger from foreign antigens, some lymphocytes immediately attack them. Certain types of lymphocytes produce substances called “antibodies.” These antibodies help find “invaders” (antigens) and, like frontline fighters, fight them. More and more lymphocytes rush to the sound of battle.
Imagine, the immune reaction is just like a small battle that plays out in your body. Usually your “self-defense army” very cleverly seeks out and destroys the enemy.
The problem is that the immune system cannot distinguish between friends and enemies. Lymphocytes don’t care whether it’s a common cold or a transplanted kidney that your body needs so much. It’s just that lymphocytes want to come to your aid, and this is where an episode of kidney rejection can develop.
When might a rejection episode begin?
In general, after you receive a kidney transplant, rejection can begin at any time. Depending on when and how a rejection episode develops, there are three types of rejection that you may have heard about from your doctors.
Hyperacute rejection occurs completely suddenly and unexpectedly. This is a very rare, but still possible event that occurs during surgery or in the first few hours after it. Transplant doctors try to avoid hyperacute rejection by carefully selecting a donor kidney to best suit the patient, and by specially preparing the donor kidney for surgery.
Sometimes after a transplant the kidney does not start working immediately. She seems to be dozing, but this is not a hyper-acute rejection.
Acute rejection is the most common type of rejection. It develops in a short time, and at first you may not feel anything at all. The risk of an acute rejection episode is highest in the first two to three months after surgery. But throughout the first year, you should be on guard for any symptoms of rejection. Acute rejection can develop after a year has passed, but even then, regular laboratory tests and necessary changes in your anti-rejection medication regimen can help prevent it.
Chronic rejection develops gradually over time. It often intensifies and sometimes leads to “failure” of the transplanted kidney. Typically, if chronic rejection develops, it develops in the first year after transplantation and can continue for months and years. Its cause is often unknown and it can be difficult to treat. The most important thing you can do to minimize the risk of such rejection is to take anti-rejection medications as prescribed by your doctor (as well as other recommended medications) and try to avoid infections and maintain good overall health.
After surgery (and sometimes before), you will be given medications to prevent rejection. They are otherwise called "immunosuppressants" or "immunosuppressants" because their purpose is to suppress your immune system so that it does not attack and damage the new kidney.
How do anti-rejection medications work?
What all transplant patients will agree on is that they have to take a ton of medications. Some of them are very important anti-rejection drugs, and each of them has its own task:
- Some medications reduce the number of lymphocytes in the immune system that can attack the new kidney.
- Others reduce the number of white blood cells (lymphocytes) by limiting the synthesis of substances needed to make new white blood cells - so-called cytostatics.
- Steroid hormones reduce inflammation, the body's natural response to injury or “intervention” (the same reaction as, for example, a skin rash if you are allergic to something).
And in some cases, in patients with an increased risk of rejection or with repeated transplantation, the use of a 4-component immunosuppression regimen is indicated: antilymphocyte antibodies (mono and polyclonal).
We list a few important points regarding the medications prescribed to you that you should not forget about.
All medications work together to prevent rejection of your new kidney. For them to fulfill their task, each of them must be taken in the right dose and at the right time, and throughout the entire time that your transplanted kidney is functioning. Some medications should be taken with food, while others should be taken on an empty stomach, depending on the doctor's instructions. Skipping even one appointment can damage the transplanted kidney and trigger an episode of rejection.
You may be prescribed other medications to treat underlying conditions, such as diabetes or heart disease. Also, because your immune system will be suppressed, you may need other medications, such as antibiotics, to prevent infections or other diseases that may cause rejection.
The surest way to avoid rejection and possible loss of kidney function is to always take all medications prescribed to you exactly as directed.
How do you know if rejection is just beginning?
Sometimes, at the very beginning of rejection, you may feel completely normal. But sometimes rejection makes itself known through certain signs. In any case, transplant doctors may be wary of several symptoms, the timely detection of which allows them to take measures to save the kidney. By monitoring your vital signs (pulse and body temperature) every day, you may notice fever, soreness in the surgical incision area, swelling of the legs or arms, decreased urine output, increased heart rate, increased blood pressure, weight gain, or feel unwell. which indicates that something is wrong in the body. Tell your transplant doctor immediately.
Routine laboratory tests contain many indicators that can tell you about your health and whether your kidney is working well. For example, one of the important indicators of kidney function is the concentration in the blood of a protein substance called “creatinine”. In itself, this substance is harmless, but if the kidney is not working well, its level in the blood will increase and serve as a signal of possible rejection.
Another test determines the concentration in your body of certain medications you take to prevent rejection. These medications are very powerful and sometimes they themselves can cause some unwanted side effects. To limit the number of side effects, your doctor will always try to prescribe you the smallest possible dose of these medications that is necessary to complete the task. However, if the concentration of the drug in the body drops too low, the immune system may rise again and begin to reject the kidney. That is why it is necessary to maintain the concentration of drugs at the desired level to prevent side effects and at the same time to prevent rejection.
The earlier an incipient rejection is identified, the easier it is to eliminate it. That is why it is extremely important to closely monitor the most important indicators of the body’s condition and regularly undergo laboratory tests.
Your transplant physicians will do everything possible to prevent any rejection episode at all. But there are a few important things you can do to help protect yourself from rejection:
Get regular check-ups and lab tests as directed by your doctor, even if you feel well or are too busy. Rejection may begin before you feel a change in how you feel. Only a doctor can detect early signs of rejection.
Monitor your basic health indicators, such as blood pressure, body temperature and weight, every day as directed by your doctor. Immediately inform him of any changes, do not hope that they will return to normal after a while. Rejection treatment is most effective when the rejection process is detected and treated early.
If you feel sick, see your doctor, even if it's just a cold, flu, stomach pain, diarrhea, or a persistent headache. Because your immune system is weak, what may be a minor illness for others may, if left untreated, cause kidney rejection for you.
Consult your doctor before taking any medicine you buy yourself without a doctor's prescription. Over-the-counter medications, vitamins, medicinal teas, herbal infusions, or dietary supplements can affect the immune system and weaken the effect of the anti-rejection medications you take. Never take medications that have been prescribed to someone else (for example, a friend or relative) or so-called “home remedies.”
Follow your doctor's advice to prevent any infections that may result in rejection. For example, wash your hands often and try to avoid contact with people who are contagious. Follow the necessary diet and exercise. This will improve your overall health and help keep your new kidney in good condition.
Take your anti-rejection medications exactly as prescribed. Never skip a dose or take a smaller dose. If you miss a dose of your medication, contact your transplant doctor right away. Only he can make any changes to the medication regimen.
What happens if the kidney fails?
Of course, loss of function of a transplanted kidney is an unpleasant event for the patient and his relatives, as well as for doctors. After all, they all had such high hopes for her.
Each organ transplant center has its own rules and procedures for retransplantation, so it is best to consult with your transplant physician if necessary.
How to form a positive attitude towards emerging problems?
Overcoming fear and anxiety about kidney transplant rejection begins with developing a positive attitude towards the problem. This means putting good intentions into action.
One of the patients who received a kidney transplant mentally turns to the cells of his immune system every morning: “Hey, buddies, this kidney is our new mutual friend. You’re not very good there with her, take it easy – and we’ll all be much better off!” In addition, he behaves very correctly: “I always follow all the instructions of my transplant doctor and follow all his advice to the last letter. This means that I:
- I take all medications exactly as I was told;
- I check the most important health indicators every day;
- I'm undergoing tests;
- I try to avoid infections and other diseases as much as possible;
- I exercise, eat well, but make sure I don’t gain too much weight.”
He says: “If you follow all these rules, then there is no need to worry about rejection anymore.
So to speak, I “rejected” the rejection. So I can stop worrying and do what really matters to me – enjoying every day of the new life that the new kidney has given me.” Thanks to modern immunosuppressive medications and improvements in treatment methods used in transplantation, many patients do not experience a single episode of rejection. If you follow your transplant doctor's instructions and lead a healthy lifestyle, you have an excellent chance of keeping your new kidney, which will give you many years of new life.