What is the procedure for taking a smear?
A smear is taken from men using a special probe or a cotton swab, which is inserted deep enough into the urethra. The patient often experiences quite unpleasant and even painful sensations. Burning and pain in the head of the penis may persist for several hours after the procedure. The material obtained using the probe is applied to a glass slide for microscopic examination. Part of the material is sent for PCR diagnostics, which makes it possible to determine the exact type of pathogen if the patient has an infectious disease.
Secondary hypogonadism in men
28.12.2020
2627
0
In continuation of the first report on primary hypogonadism in men, andrologist, urologist, head of the department of andrology and urology of the National Medical Research Center for Obstetrics, Gynecologists and Perinatology named after V.I. Kulakova, Professor Safar Israidovich Gamidov gave an extensive lecture on the features of secondary hypogonadism in men and available treatment regimens.
S.I. Gamidov
Doctor of Medical Sciences, Professor, Federal State Budgetary Institution “National Medical Research Center for Obstetrics, Gynecology and Perinatology named after. Academician V.I. Kulakov" (Moscow)
As Safar Israilovich recalled, it is mandatory for an andrologist to understand the physiological role of hormones, the ability to interpret endocrine indications for diagnostic purposes, as well as prescribe hormonal drugs with the expected therapeutic effect.
There are a number of cases when an andrologist should think about prescribing hormonal therapy:
- pediatric urology: cryptorchidism, hypospadias, micropenis;
- hypogonadism, including with associated erectile dysfunction;
- hyperprolactinemia;
- infertility (oligozoospermia, sperm DNA fragmentation);
- the need for male contraception;
- priapism;
- benign prostatic hyperplasia;
- prostate cancer with unfavorable prognostic features.
The reproductive function in men works thanks to the hypothalamic-pituitary-gonadal system. In the hypothalamus, the infundibular nucleus is important, containing KNDy neurons (a subpopulation of arcuate nucleus neurons) and GnRH neurons. In the anterior pituitary gland, gonadotrophs produce luteinizing hormones (LH) and follicle-stimulating hormones (FSH). In turn, the gonads produce sex steroids and gametes under the influence of LH and FSH.
Testosterone, estradiol, LH, FSH, prolactin, activins and inhibins, as well as anti-Mullerian hormone play an important role in the regulation of the hypothalamic-pituitary-gonadal system.
In 1977, the groups of A. Schally and R. Guillemin were awarded the Nobel Prize for the discovery of GnRH (gonadotropin-releasing hormone, GnRH). It is a fairly simple decapeptide. At first it was called “LHRH”, but then it turned out that it regulates the secretion of not only LH, but also FSH. As Safar Israilovich explained, a person has on average only 1000–1500 GnRG neurons. They produce GnRH, which enters the portal vein system of the hypothalamic-pituitary apparatus. GnRH circulates in the coronary network of the hypothalamus and pituitary gland.
In women, pulse secretion and GnRH releases play an important role in the regulation of the menstrual cycle. Healthy men have only one mode of its secretion - pulse secretion. About 10–20 secretory impulses occur per day, and there are no pacemakers who could clearly regulate them. The secretion of gonadotropins (LH and FSH) from the anterior pituitary gland is stimulated by the pulsatile release of GnRH and inhibited by the release of gonadotropin-inhibiting hormone from the hypothalamus. LH and FSH have a common a-subunit and a separate ß-subunit. LH stimulates and increases the production of testicular androgen by Leydig cells, and FSH stimulates spermatogenesis in Sertoli cells [1–3].
FSH is a useful indicator for assessing the functional state of the male germinal epithelium, although it is far from perfect. Despite the wide range of standard values (1.4–18.1 IU/l), according to some authors, values of this hormone > 4.5 IU/l already indicate a high risk of impaired spermatogenesis. In addition, unstimulated FSH is a poor predictor of successful sperm surgery in men with non-obstructive azoospermia (NOA) [4, 5].
In practice, mutations of the FSH and LH receptors occur. Mutations of the FSH-ß gene in men invariably lead to azoospermia and a decrease in testicular size. According to some scientific data, existing mutations in FSH receptors provide varying degrees of effects on male fertility. Moreover, depending on the nature of the mutation, the severity of changes in spermatogenesis can manifest itself from severe oligozoospermia to normozoospermia with the possibility of conception naturally. These clinical data correlate well with experimental results obtained in mouse models. Mouse models with altered LH receptors demonstrate decreased levels of serum and intratesticular testosterone and the occurrence of a late block in spermatogenesis [3, 6, 7].
An important factor in the formation of fertility is the level of testosterone. In particular, a large prospective cohort study of 357 men with idiopathic infertility and 318 fertile men found that infertile men had significantly lower levels of total and free testosterone, testosterone/LH ratio, and testosterone/estradiol (p < 0.001) [8].
The main known endocrine regulators of spermatogenesis include testosterone (regulates the number of pale spermatogonia, the effect on other stages of spermatogenesis has not been proven) and FSH (the main factor regulating all stages of spermatogenesis, except meiosis). From a clinical point of view, the synergism of testosterone and FSH is necessary for the initiation and maintenance of active spermatogenesis [3].
With regard to androgen receptors, immunohistochemical analysis of testicular tissue from infertile men showed isolated distribution of androgen receptors only to Sertoli cells. Expression of these receptors in Sertoli cells and adequate intratesticular testosterone concentrations are required for both completion of germ cell meiosis and spermatogonia proliferation. Damage to androgen receptors in Sertoli cells in mice leads to early arrest of spermatogenesis. Insufficient intratesticular testosterone concentrations are associated with impaired integrity of the blood-testis barrier in mice. Increasing the dose of testosterone and FSH promotes the in vitro development of germinal cells obtained from men with early and late block of spermatogenesis into spermatids, and this allows the ISCI procedure to be performed [9–12].
Testicular aspiration biopsy studies in fertile men have confirmed that testosterone is the predominant intratesticular androgenic hormone. The intratesticular testosterone concentration is 60,000 ng/dL, which is much higher than the average serum testosterone concentration in men (500 ng/dL). In the study, intratesticular testosterone concentrations exceeded SHBG concentrations, indicating that the majority of intracellular testosterone is likely in a bioavailable form [13].
Using new analytical methods, the same authors showed that 70% of all intratesticular testosterone is a biologically active substance. However, the concentration of total serum testosterone weakly correlated with the concentration of intratesticular testosterone (p = 0.38). According to new tests, serum bioactive androgens also weakly correlate with intratesticular levels of bioactive androgen (r = 0.46; p = 0.03).
Serum and intratesticular testosterone levels measured simultaneously were highly correlated across studies (r = 0.67, p = 0.03). In addition, significant variability in intratesticular testosterone levels was found, which was highly correlated with serum LH (r = 0.87; p = 0.01). According to the authors, pulsatile LH secretion may reflect a similarly pulsatile secretion of intratesticular testosterone. Pulsatile secretion of intratesticular testosterone was also confirmed when assessing the concentrations of testosterone and estradiol in cannulated gonadal veins in men with varicocele. Sampling every 15 min revealed hourly testosterone pulses (3-5 pulses per hour) with a mean amplitude of 176 +/- 42 ng/mL, 6.6 times the nadir. However, today the effect of intratesticular pulsatile testosterone secretion on spermatogenesis is unknown [14]. However, the same experimental work showed that spermatogenesis in the testicle persists until the level of intratesticular testosterone falls below 75% of the baseline concentration. However, the exact level of intratesticular testosterone at which human spermatogenesis is impaired is not fully clarified.
A promising biomarker of intratesticular androgen status is insulin-like protein 3 (INSL3), which is secreted by mature Leydig cells. Testicular aspiration and serum analysis confirmed that INSL3 was closely correlated with intratesticular testosterone both during induced hypogonadism and after hCG treatment (r = 0.79, p < 0.001). However, inhibin B and anti-Mullerian hormone did not correlate with intratesticular testosterone. Despite these promising results, INSL3 is not yet used in routine clinical practice as a biomarker of Leydig cell function and intratesticular androgen status [15].
An important indicator of the degree of spermatogenesis is inhibin B. In the case of hypospermatogenesis, its secretion decreases, and the secretion of FSH increases. One prospective study found that inhibin B and FSH were significantly correlated with vital semen parameters. At the same time, the values of the correlation coefficient for inhibin B are higher than for FSH (inhibin B: 0.48; p < 0.0001, FSH: 0.41; p = 0.0007) [16].
Activin has the opposite effect. It is a heterodimer or homodimer of ß subunits and is also produced in the testes. Activin has an agonistic effect on the secretion of FSH by the pituitary gland, and its release is inhibited by inhibin B. Activin is not currently used in the clinical assessment of male fertility [17].
In 2001, the peptide kisspeptin was discovered. It has an agonistic effect on GnRH release and pulsatile LH release. Kisspeptin has been used experimentally in men to induce gonadotropin secretion. It has been experimentally shown that the administration of kisspeptin is accompanied by a more pronounced increase in LH than FSH. Currently, kisspeptin is not used in clinical practice [18].
Estradiol is produced from testosterone by aromatase, a member of the cytochrome P450 family. The concentration of estradiol in the blood is low in men, but extremely high in sperm, and its content in the testicle can reach 250 pg/ml, which is higher than in the serum of women. According to available scientific data, only the use of testosterone and estradiol leads to a decrease in the levels of LH and FSH in the blood serum. The inhibitory effect of estradiol on LH is due to a decrease in sensitivity to GnRH. Dihydrotestosterone, which is not converted to estradiol, did not have an inhibitory effect on gonadotropin secretion [19, 20].
For normal spermatogenesis, a sufficient level of estradiol in the testicle is necessary, which has been proven by clinical studies involving infertile men with congenital aromatase deficiency. Estradiol receptors are expressed in Sertoli and Leydig cells, as well as in germ cells. Experimental animals with damaged estradiol receptor are infertile and have abnormal spermatogenesis. Estrogen downregulates spermatogenesis-related genes and induces spermatocyte apoptosis through estrogen receptors 1 and 2. Estrogen regulates luminal fluid reabsorption in the caput epididymis. According to available data, infertility in this category of animals occurs due to impaired reabsorption of fluid from the proximal epididymis and a subsequent increase in intraluminal pressure. This finding raises concerns about the potential direct effects of environmental androgens on male reproduction and reports of decreased sperm count in humans [21–23].
Also involved in the reproduction of aromatase are cytochrome P450 enzymes, which irreversibly convert androgens to estrogens in various tissues, including the testicle, liver, brain and adipose tissue [21, 24]. In the testis, aromatase is localized in Leydig cells [25]. A large retrospective cohort study found an association between infertility, vital semen parameters and reduced testosterone/estradiol ratios. This ratio <10 was considered to be the 20th percentile cutoff point among fertile men. In particular, a similar decrease in the testosterone/estradiol ratio is observed in obese men due to increased peripheral aromatization [26].
Sertoli cells also express thyroid hormone receptors. In addition, thyroid hormones influence the development of Leydig cells and steroidogenesis. Thus, thyroid hormones can maintain different populations of germ cells. There is a connection between teratozoospermia and the presence of a clinical picture of hyper- or hypothyroidism [27, 28].
Under physiological conditions, with a sharp increase in the concentration of prolactin in the blood, testosterone secretion may increase. Many researchers believe that the sleep-related peak in testosterone secretion in men is partly due to an increase in plasma prolactin levels. With chronic hyperprolactinemia in men, GnRH impulses are inhibited, and, consequently, the secretion of gonadotropins and the level of testosterone in the blood are reduced [29].
As Safar Israilovich recalled, there are several types of regulation in the body. Autocrine regulation - the presence of receptors for GnRH, cannabinoids and opioids in the hypothalamus. Paracrine regulation - the presence of receptors for GnRH II and LH, which retrogradely enter the hypothalamus through the portal blood flow. Endocrine regulation - testosterone and estradiol close the negative feedback loop on the pituitary gland and hypothalamus, respectively.
Regulators of GnRH neuron activity in general include estradiol, progesterone, dihydrotestosterone, testosterone, estradiol, cortisol, prolactin, gamma-aminobutyric acid, activins and inhibins, endocannabinoids, opioids, serotonin, leptin and melatonin.
Hypogonadotropic hypogonadism is associated with a deficiency in the production of GnRH and LH/FSH. It can be congenital (with or without anosmia) and acquired (traumatic brain injury, neuroinfections, tumors, operations). Isolated LH deficiency (Pascalini syndrome) is very rare, and isolated FSH deficiency is casuistically rare [30].
Intracranial tumors are the most common cause of acquired forms of hypogonadotropic hypogonadism. Unlike acquired forms, the etiology of congenital hypogonadotropic hypogonadism is more complex and in most cases is idiopathic in nature. The prevalence of congenital hypogonadotropic hypogonadism in men is 1/10,000 [31].
Up to 90% of cases of secondary hypogonadism are not accompanied by signs of organic damage to the testicles or other components of the hypothalamic-pituitarygonadal axis. For these situations, the term “functional hypogonadism” has been proposed [32, 33]. Up to 85% of men with sexual dysfunction due to low testosterone levels have signs of metabolic syndrome.
The list of medications used for hypogonadism is quite wide:
- serotonin reuptake inhibitors;
- antiarrhythmic drugs (amiodarone);
- anticonvulsants (phenytoin);
- antifungal drugs (ketoconazole);
- opiates;
- antipsychotics;
- statins in high doses;
- thiazide diuretics;
- spironolactone;
- cimetidine
To differentiate hypogonadotropic hypogonadism from the constitutional form of delayed puberty, it is necessary to take into account the results of the following tests:
- physical examination;
- karyotype analysis;
- determination of the level of sex hormones;
- test with GnRH analogue;
- determination of bone age;
- MRI of the hypothalamic-pituitary region.
A number of main goals and objectives of the treatment of hypogonadotropic hypogonadism are identified. Thus, the goals of hormonal therapy in this case are to maintain testicular function, develop secondary sexual characteristics, improve quality of life and restore reproductive function. The key to successful treatment of hypogonadotropic hypogonadism is the choice of an adequate drug with an appropriate dose. Preparations of testosterone, gonadotropins and GnRH with a pulsatile pump can be used in the hormonal regulation of patients with hypogonadotropic hypogonadism. If it is necessary to preserve reproductive function in this category of patients, gonadotropins are most preferable [34].
When treating hypogonadotropic hypogonadism, hCG 1000–1500 units. 3 times a week or 2500 units. 2 times a week for 3-6 months, the main goals are normalization of testosterone levels, transition of dark A cells to pale A cells and proliferation of the germinal cell pool. During FSH therapy, 75–150 units. 3 times a week (recombinant, purified or as part of menopausal gonadotropin), the injection area should not coincide with hCG. FSH stimulates the transformation of pale A cells into B cells and subsequent meiosis, and initiates spermatogenesis [35].
Spermatogenesis usually recovers within 6–9 months, although in some patients the process can take up to two years. In approximately 10% of patients, despite therapy using hCG and FSH, spermatogenesis is not restored at all [36]. The effectiveness of therapy does not depend on the etiology of hypogonadotropic hypogonadism: only testicular volume allows predicting the result [37]. The threshold value for testicular volume to predict the effectiveness of gonadotropin therapy is 4 cm3 [38].
In particular, in a study of 36 men from 11 to 42 years old with hypogonadotropic hypogonadism (81% - primary and 19% - secondary), long-term therapy with chorionic gonadotropin drugs was practiced - from 12 to 240 months (average 56). An increase in the number of sperm among patients with reduced testicular size was noted in 36% of cases, and with normal testicular size, an increase in sperm concentration was noted in 71% of patients.
Some authors today mistakenly believe that hCG is an analogue of LH. This is wrong. In particular, oligosaccharide chains protect hCG from biodegradation. The development of immune-mediated resistance to hCG is also possible [39]. In general, combination therapy with hCG and FSH achieves greater success than monotherapy. Side effects of this treatment included acne and gynecomastia.
According to one of the available studies of ten years of experience using hCG and MCG separately and together for the treatment of patients with hypogonadotropic hypogonadism, during combination therapy, compared with hCG, an increase in testicular volume was significantly more often observed (75 versus 50%, respectively). 86% of patients receiving the combination treatment eventually achieved spermatogenesis, compared with 81% of those receiving hCG alone [40]. In approximately 10% of cases, hypogonadotropic hypogonadism resolves spontaneously after stopping the combination treatment. Risk factors for treatment failure include cryptorchidism and small testicular volume (less than 4 ml).
An alternative way to stimulate spermatogenesis in this category of patients may be a pulsatile infusion of GnRH. Hoffman and Crowley in 1982 used gonadorelin as a subcutaneous pulse infusion in patients with hypogonadotropic hypogonadism and achieved spermatogenesis induction in them [41, 42]. Also Chinese researchers in 2010 and 2011. reported the successful use of pulsed subcutaneous infusion of GnRH to trigger spermatogenesis in hypogonadotropic hypogonadism, but the sample size of these studies was relatively small [43, 44].
Pulse therapy with GnRH analogs requires the installation of a subcutaneous or venous pump and mimics the natural pulse secretion of GnRH, requiring regular cannula changes every 2–3 days. This method is considered justified only for hypogonadotropic hypogonadism with azoospermia. Compared with hCG + FSH therapy, this method requires less time to restore spermatogenesis, with it there are fewer testosterone peaks, a lower incidence of acne and breast pain, but a higher incidence of local skin reactions [43].
In 2021, the CONSORT randomized controlled trial was undertaken to comparatively evaluate the effectiveness of hormonal therapy for hypogonadotropic hypogonadism in different regimens, involving 220 patients. Participants were divided into four groups. Group 1 - GnRH for congenital hypogonadotropic hypogonadism, group 2 - GnRH for acquired hypogonadotropic hypogonadism, group 3 - hCG/MCG for congenital hypogonadotropic hypogonadism and group 4 - hCG/MCG for acquired hypogonadotropic hypogonadism. Efficacy assessment was carried out after 18 months [45]. The GnRH group received subcutaneous infusions of gonadorelin at a dose of 100 to 500 mg per day using a special pump every 90 minutes (16 pulses per 24 hours). Depending on the level of FSH, LH and testosterone, the dose of the drug was adjusted. Simultaneously, a solution of hCG at a dose of 5000 IU and MCG at a dose of 75 IU were used. The dose of MHC ranged from 75 to 150 IU in intramuscular injections once or twice a week. The dose of hCG is from 5,000 to 10,000 IU intramuscularly once or twice a week. The dosage of the drugs was adjusted depending on the testosterone level and spermatogenesis productivity, and the testosterone level was maintained at 10–15 nmol/l. The authors showed that either approach to therapy was effective: 25 months after the start of therapy, 90% of patients achieved at least a minimal presence of sperm in the ejaculate, but sperm concentrations of 10–15 million/ml were achieved in only 50% of men. Patients who achieved higher peak LH levels during therapy had a higher rate of sperm recovery in the ejaculate.
In some cases, patients may be prescribed selective estrogen receptor modulators (SERMs), but a common mistake is the unnecessary use of such drugs, in particular clomiphene. In true hypogonadotropic hypogonadism, there is no substrate for SERM activity. In the case of secondary pituitary hypogonadism, there are no pituitary gonadotrophs, and there are no cells capable of synthesizing LH and FSH. Hypothalamic hypogonadism is “tertiary”, in which there is no point in eliminating negative feedback if there is no positive stimulating factor (GnRH). Finally, in hypogonadism associated with hyperprolactinemia, GnRH production does not normalize unless excess prolactin levels are corrected. When prolactin levels are elevated, in particular, the use of cabergoline can significantly improve patients' hormonal levels and help restore sexual function [46].
In conclusion, Safar Israilovich recalled that the hypothalamic-pituitary-gonadal axis is a fragile structure, the functioning of which is better not to interfere without reason and a clear understanding of your actions. The best treatment for hypogonadism in men who are not interested in preserving their fertility is testosterone replacement therapy. Monotherapy with hCG in most cases, with some exceptions, is unjustified and carries risks. It should be remembered that both testosterone and FSH are necessary for normal spermatogenesis. Finally, in case of hypogonadism and erectile dysfunction, it makes sense to conduct a test for serum prolactin.
Sources:
- Ubuka T et al. Front Endocrinol, 2014
- Kumasi TR. Cell Endocrinol, 2007
- Layman LC et al. J Clin Endocrinol, 2002
- Kwee J et al. Fertil Steril, 2008
- Ramasamy R et al. J Urol, 2013
- Kumar TR. Molec Cell Endocrinol, 2007
- Siegel et al. Reprod Sci, 2013
- Andersson AM et al. J Clin Endocrinol Metab, 2004
- Tesarik J et al. Fertil Steril, 2002
- Kato Y et al. Andrology, 2014
- De Gendt K et al. A Proc Nat Acad Sci USA, 2004
- Cheng CY et al. Prarmacol Rev, 2012
- Jarow JP et al. Ann NY Acad Sci, 2005
- Roth MY et al. J Androl, 2010
- Roth MY et al. Fertil Steril, 2013
- Meacham SJ et al. Euro J Endocrinol, 2001
- Hedger, MP et al. Mol Cell Endocrinol, 2012
- Skorupskaite K. Hum Reprod Upd, 2014
- Bagatell C et al. J Androl, 1994
- Hayes FJ et al. Clin Endocrinol Metab, 2000
- Carreau S et al. Adv Med Sci, 2012
- Hess RA et al. Nature, 1997
- Eddie E.M. et al., 1996
- Schlegel PN. Fertil Steril, 2012
- Inkster S.J. J Clin Endocrinol Metab 1995
- Saylam B et al. Fertil Steril, 2011
- Mintziori G. Endocrinol Invest, 2016
- Kumar A. Indian J Endocrinol Metab, 2014
- Corsello SM et al. Clin Endocrinol, 2003
- Mao JF et al. Asian J Androl, 2017
- Varimo T et al. Hum Reprod, 2017
- Grossman, Matsumoto, 2017
- Corona, Maggi, 2015
- Lin et al. Medicine, 2019
- Arroyo et al., 2015
- Schiff et al., 2007
- Farhat et al., 2010
- Miyagawa Y, Tsujimura A. J Urol, 2005
- Leao, Esteves, 2014
- Yang L. Int Urol Nephrol, 2012
- Belchetz PE et al. Science, 1978
- Dwyer AA. Best Pract Rec Clin Endocrinol Metab, 2015
- Zhang G.Y. Reprod and Contracept, 2010
- Sun Y et al. Clin J Endocrinol Metab, 2011
- Lin et al. Medicine, 2019
- De Rosa M et al. J Clin Endocrinol Metab, 2004
The material was prepared by Shaderkina V.A. The video can be viewed on Uro.TV
Comments
To post comments you must log in or register
How is the results of a smear for flora assessed in men?
The smear material may contain the following components:
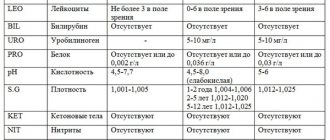
- Leukocytes - in a smear in men in plural numbers, they are a sign of inflammation and speak of urethritis or prostatitis. Normally, a smear should not contain more than 5 leukocytes in the field of view.
- Epithelium is the cells of the urethral mucosa. Normally, 5–10 cells are allowed per field of view. A larger number of them indicates inflammation.
- Mucus – occurs in large quantities during inflammatory processes.
- Cocci - a small number of them are normal, since they are part of the normal microflora of the mucous membrane. Many cocci in the field of view are a sign of urethritis.
- Gonococci, trichomonas, ureaplasma, chlamydia and other bacteria, protozoa are normally absent from the smear. If they are found, a diagnosis of the corresponding sexually transmitted disease is made.
In some cases (for example, with chronic prostatitis), before taking a smear for flora, a prostate massage is performed through the rectum.
Expert: Dmitry Grishin, urologist Author: Ekaterina Khripunova
Symptoms
Symptoms that are indicative of a mandatory smear:
- Feelings of pain, heaviness and discomfort observed in men in the genital area.
- Complaints of burning and itching in the urethra.
- The appearance of a rash on the penis.
- Frequent, painful urination and other related problems.
- Observation of pathological discharge from the urethra.
- The appearance of swelling in the genital area and groin, the appearance of discharge from the penis or blood in the urine and semen.
If you have unpleasant symptoms, you should immediately visit a doctor, take a smear and get tested for the presence of sexually transmitted infections. What epithelium means in a smear depends on the characteristics of its cells and their structure and quantity.
Why is columnar epithelium detected in a smear in men? We will tell you about this further.
What is determined by a urethral smear? Various sexually transmitted diseases, the presence of pathogens, progression of the inflammatory process.