Author
— Douglas S. Kalman, Doctor of Nutrition.
Creatine monohydrate has been used as an energy support since the early 70s. In America, its use boomed in the 90s. This is fully consistent with recent research showing that creatine monohydrate supplementation has an ergogenic effect (increases athletic performance). According to one recent study, 37.5% of US male college athletes use a creatine monohydrate supplement. Based on sales results and well-known effectiveness, it is safe to say that creatine monohydrate is by far the most popular dietary supplement among strength athletes.
Creatine is found in foods and can be synthesized by the liver, pancreas and kidneys. The daily turnover of creatine in the body of a 70-kg person is 2 grams. The body synthesizes approximately half of the daily creatine requirement from amino acids. The other half comes from food. Fish and meat are the best natural sources of creatine. For example, 250 grams of raw lean beef contains 1 gram of creatine. However, the most common way to load muscles with creatine among athletes is through dietary supplements, that is, synthetic creatine monohydrate.
Research Review
Creatine exists in the body in free form and in the form of phosphate forms, that is, phosphocreatine or creatine phosphate. Mostly (up to 95%) creatine is found in muscles and is used as a buffer. When we exercise, the body experiences a sharp increase in energy needs. During periods of increased energy demand, phosphocreatine produces phosphate for adenosine diphosphate (ADP) so that adenosine triphosphate (ATP), the body's energy currency, can be produced. Exercises that require a short burst of energy depend on both ATP and phosphocreatine as energy sources.
Figure 1 shows during which phase of exercise creatine is most needed. Supplying the body with creatine monohydrate increases creatine phosphate stores (and circulating levels of creatine). Thus, a person taking a creatine monohydrate supplement and training at high intensity will receive "extra" ready-made creatine. Consequently, his body will be able to train more actively and recover faster.
Figure 1. Dominant energy pathways of different training intensities.
Class time.
ATP - light gray Creatine - dark gray Anaerobic glycolysis - black Aerobic glycolysis - white.
Creatine monohydrate has been commonly used by athletes and in loading and maintenance studies. An example of a loading regimen is consuming 20 grams of creatine per day for five to seven days. This loading format will increase muscle creatine content by 25 percent. A recent study found that consuming 2 grams of a creatine monohydrate supplement daily for 30 days would result in the same increase in muscle creatine content as a five-day supplement. This means that in some cases (for example, when there is no need for a sharp increase in creatine levels), creatine loading may not be necessary, and it is better to take a maintenance dose (up to 5 grams) of creatine per day.
The absorption of creatine monohydrate into muscles and its intake mainly depend on the initial level of creatine in the muscles. Control groups with the lowest starting creatine levels absorbed the most creatine monohydrate. It was also found that the absorption of creatine monohydrate is better when combined with 50 grams of a carbohydrate-protein supplement.
Creatine monohydrate supplementation may be less effective in the following cases:
- when a dose of less than 20 grams is taken for less than five days (no loading);
- when small doses (2-3 grams per day) are taken without preliminary, intensive loading for a short time;
- in studies that are not long enough (less than 5 weeks);
- in the case where sprint races were carried out with a very short or very long recovery period.
It is also possible that differences in how subjects responded to creatine monohydrate may be due to a lack of ergogenic benefit in these studies. In the vast majority of cases, supplementation with creatine monohydrate leads to improved performance, but this does not mean that creatine monohydrate “works” and provides a positive effect for absolutely all athletes.
In laboratory studies, creatine monohydrate taken as a dietary supplement has been found to have an ergogenic effect in the following situations: repeated sprints on a stationary bike, weight training, leg extensions, rowing, swimming and rugby. Maybe in some sports creatine monohydrate does not work as effectively as in those listed above. It is important to remember that creatine monohydrate provides an ergogenic effect only in those sports where physical effort lasts from 2 to 90 seconds or is repeated several times in a row.
CREATINE
Creatine
(syn.:
methylglycocyamine, guanidine-methylglycine
) - methylguanidine acetic acid, is one of the important components of nitrogen metabolism in the body;
accumulating in tissues in the form of a highly energetic phosphorylated derivative - creatine phosphate, Creatine also participates in energy metabolism. Creatine plays a regulatory role in many biochemical processes: it stimulates the biosynthesis of proteins (creatine kinase, actin and myosin heavy chains), activates the process of respiration and oxidative phosphorylation in mitochondria. creatine
Processes that occur in the cell with energy consumption - muscle contraction, active transport of ions in nervous tissue - are accompanied by the breakdown of significant amounts of creatine phosphate and the accumulation of creatine at a constant level or small changes in the concentration of ATP.
In medicine, determination of the content of Creatine and its derivative, creatinine, in the blood and urine serves to diagnose a number of diseases.
Creatine is found in various tissues of humans, vertebrates and some species of invertebrates. It was first discovered by the French. scientist Chevreul (M. E. Chevreul) in 1835 in an extract from skeletal muscles.
Mol. weight (mass) K. 131.14. K. forms crystals with the addition of one molecule of water, having the form of monoclinic prisms (mol. weight 149.16). Relatively poorly soluble in water (1.35 g in 100 g of water at 18°), insoluble in ether, very poorly soluble in alcohol (0.0063 g in 100 g of cold ethanol).
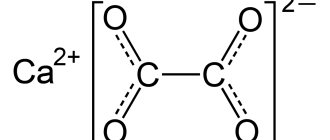
In a strongly acidic environment, calcium loses a particle of water and turns into creatinine by closing the bond between the NH2 and COOH groups.
creatinine
This property of carbon is the basis for one of the methods for its quantitative determination.
Creatinine (1-methylglycocyamidine) is one of the end products of protein metabolism in vertebrates and humans and is constantly present in urine. The amount of creatinine secreted by a person per day is on average 0.6-2 g and depends on the degree of muscle development and the content of creatine phosphate in it. The ratio of the amount of creatinine (in mg) excreted by a person per day to body weight (in kg) is called the creatinine ratio. Typically, the creatinine ratio ranges from 20 to 30 for men and 10 to 25 for women.
In the urine, along with creatinine, K is also found. In healthy adults, the content of K in the urine is very small (0.05-0.25 g in the daily volume of urine). The release of significant amounts of K. in the urine is called creatinuria and is observed in various pathological conditions, as well as in normal children (see Creatinuria).
In the skeletal muscles of humans and vertebrates, the total amount of calcium is (in mg%) 250-550; in the heart muscle -150-300; in smooth muscles -50-100; in brain tissue - 100-150. In the organs where K. is synthesized—the kidneys, liver, and pancreas—the K. content is very low (10-40 mg%). In small concentrations (1-1.5 mg%) K. is found in the blood plasma of humans and animals (see Creatinemia).
In the body of animals and humans, K. is synthesized from three amino acids: arginine (see), glycine (see) and methionine (see). K synthesis occurs in two stages. The first stage of synthesis - the formation of guanidine acetic acid by transferring the amidine group from arginine to glycine - occurs in the kidneys and pancreas with the participation of the enzyme L-arginine: glycine amidinotransferase (EC 2.6.2.1). The activity of this enzyme in the pancreas is 5 times greater than in the kidneys.
The second stage of Creatine synthesis - the methylation reaction of guanidine acetic acid with the participation of the activated form of methionine (S-adenosylmethionine) occurs in the liver and pancreas. This reaction is catalyzed by the enzyme guanidine acetate methyltransferase (EC 2.1.1.2).
It is believed that from the liver and pancreas K. passes through the bloodstream into various organs and tissues (skeletal and cardiac muscles, brain and nervous tissue).
The pathways for the enzymatic breakdown of cellulose in the tissues of vertebrates and humans are unknown. About 2% of the total K content in the body is non-enzymatically converted into creatinine every day, which is excreted in the urine.
In muscle and brain tissues, with the participation of the enzyme creatine kinase (see), K. enters into a transphosphorylation reaction with ATP, thereby turning into creatine phosphate. This is the only known pathway for the formation of creatine phosphate.
Creatine phosphate was described in 1927 by P. Eggleton and QP Eggleton, S. H. Fiske and Y. Subbarow. In 1929, Fisk and Sabbarow established that this compound consists of potassium and phosphorus in a molar ratio of 1:1.
creatine phosphate
Creatine phosphate belongs to the class of phosphagens (see) - highly energetic phosphorylated derivatives that play the role of energy accumulators in the cell. Under physiological conditions, the free energy of hydrolysis of the phosphoamidine bond of creatine phosphate is 10.3-10.8 kcal/mol. Creatine phosphate, along with K. and creatine kinase, is found in almost all organs and tissues of vertebrates and humans and some species of invertebrate animals (echinoderms, chordates). The largest amount of creatine phosphate is found in skeletal muscles. Back in 1922, A.V. Palladin suggested the important role of calcium in the chemistry of muscle activity. A.V. Palladin and his co-workers. It has been shown that during training, which causes an increase in muscle performance, the latter are always enriched with calcium and creatine phosphate. As muscle function weakens, the creatine phosphate content decreases.
It has been established that creatine phosphate regulates glycolysis (see), inhibiting phosphofructokinase, pyruvate kinase and activating glucose-1,6-diphosphatase. See also Nitrogen metabolism.
Methods for determining creatine in biological fluids. K. is determined after preliminary conversion to creatinine by heating the test sample with mineral acid. Creatinine in blood and urine is determined by the Folin-Popper method, based on the reduction of picrine acid by creatinine in a highly alkaline medium to picramic acid, which has a red-orange color (see Jaffe reaction).
Determination of K. is also carried out using a method based on the formation of a colored compound K. with diacetyl and alpha-naphthol in an alkaline medium. Determination of the concentration of the colored complex is carried out colorimetrically or spectrophotometrically at 540 nm.
Creatine phosphate is determined by creatinine or phosphorus. The first method consists of the decomposition of creatine phosphate into creatinine and phosphoric acid in the presence of ammonium molybdate in an acidic environment and the colorimetric determination of creatinine with picric acid. The second method is based on the breakdown of creatine phosphate in an acidic environment and the subsequent determination of the resulting mineral phosphate with molybdenum acid and eiconogen.
More sensitive is the spectrophotometric method for determining K. with creatine kinase and two enzyme systems associated with it - pyruvate kinase and lactate dehydrogenase (Tanzer-Gilvarg method). The method is based on the fact that when ATP and creatine kinase are added to the test sample, all endogenous creatine is converted into creatine phosphate and an equimolar amount of ADP is formed. The concentration of the resulting ADP is determined in the presence of pyruvate kinase, lactate dehydrogenase and their substrates added to the sample spectrophotometrically by the decrease in the absorption of reduced NAD-H2 at 340 nm.
The most sensitive method for determining K. in biological liquids is the fluorimetric method, based on the formation of a complex of K. with ninhydrin in an alkaline medium, which has a fluorescence maximum at 495 nm. By this method, K. is determined in a concentration of 1 • 10-7 M. Guanidine and some of its derivatives, which are usually found in urine, interfere with the determination of K. Guanidine is effectively removed from urine when it is treated with anion-exchange resins.
Bibliography:
Mardashev S. R. Biochemical problems of medicine, p. 110, M., 1 975; Palladin A.V. Selected works, p. 109, Kyiv, 1975; TodorovY. Clinical laboratory research in pediatrics, trans. from Bulgarian, p. 169, 739, Sofia, 1968; Clinical biochemistry, ed. by H.-Ch. Curtius a. M. Roth, v. 2, B.—NY, 1974.
L. B. Belousova.
Side effects
The only side effect of creatine monohydrate supplementation in pre- and post-surgical patients, untrained individuals and elite athletes was weight gain. However, from time to time, reports appear in periodicals and on the Internet about alleged side effects of using creatine monohydrate supplements. However, they are not supported by any research or experimental data. However, unfortunately, some of these pseudoscientific “concerns” have received some press coverage recently, creating the illusion that there is room for debate on this issue.
Since creatine is an amino acid, it has been suggested that its consumption may negatively affect liver and kidney function. However, no research data were provided to support the occurrence of liver or kidney dysfunction as a result of the use of creatine monohydrate supplements. All these are just words. Not a single study has revealed any side or negative effects from the use of creatine monohydrate by athletes in summer sports (competitions take place outdoors).
It has also been reported several times that creatine monohydrate may cause an increased risk of muscle strains. But no specific data was ever provided on this matter.