The role of Uro-vaxom in the complex treatment of patients with chronic pyelonephritis and cystitis
AND
Infectious and inflammatory diseases of the genitourinary system occupy an important place in the structure of urological pathology, especially among women.
80–90% of all patients with chronic pyelonephritis and cystitis are women; 10–20% of women experience an episode of urinary tract infection at least once during adulthood [8,14]. Despite the huge number of powerful antibiotics, the problem of treating patients with urinary infections is far from being resolved. First of all, we see the reason for this as the lack of an integrated approach. The doctor discovered bacteriuria and leukocyturia in the patient; coupled with complaints of pain in the kidney area, frequent painful urination, rise in body temperature, etc. this is certainly an indication for treatment. What goal does the doctor set for himself in such a situation? Achieve eradication of the pathogen and, indirectly, stop inflammation. And the achievement of this goal, judging by domestic and foreign publications, is expected by prescribing antimicrobial drugs. Is it possible to destroy a microbial agent with antibacterial therapy in the case of uncomplicated acute or exacerbation of chronic nonspecific pyelonephritis or cystitis? Yes, in the vast majority of cases. Can we talk about the patient’s recovery? Not always. Of course, in a situation of acute cystitis, provoked by some obvious single action, taking fosfomycin can stop dysuria and sanitize the urine within 24 hours. It is likely that in the absence of repeated provocation the patient will never develop cystitis again. In this case, recovery occurred as a result of taking an antibiotic. Unfortunately, circumstances usually turn out differently. Much more often, a patient comes to the appointment who has received many courses of antibacterial therapy, both for genitourinary tract infections and for concomitant diseases. What decision will the average doctor make? Prescribe a stronger antibiotic at the maximum dosage. Will he achieve the destruction of the pathogen? Very likely, yes. Will he cure the patient with this? Most likely not. The patient we talked about above was treated with antibiotics for a long time. It can be said with 100% certainty that she has intestinal and vaginal dysbiosis and a disturbed immune response. There is a high probability of presence in the urine, in addition to microbial flora, also of fungi. The long course of the infectious-inflammatory process in such unfavorable conditions actually transforms an uncomplicated urinary tract infection into a complicated one
, as it promotes the selection of resistant pathogens and disrupts the natural passage of urine.
Thus, the use of antibacterial therapy alone is not enough; a complex pathogenetic effect
.
Pathogenetic measures should include laser therapy (with strict consideration of contraindications), infusion, antioxidant, and anti-inflammatory therapy. In addition, you should use the drug Uro-vaxom
, which is actually a means of etiopathogenetic treatment of urinary infections.
Uro-vaxom is a 6 mg lyophilized bacterial lysate of 18 different strains of E. Coli
, enclosed in a gelatin capsule for daily administration on an empty stomach once a day.
Since E. Coli
is the main causative agent of both complicated and uncomplicated urinary infections [2,3], the targeting of Uro-Vaxom can be regarded as an etiotropic effect.
However, the inhibitory effect of Uro-vaxom on both S. aureus
and
C. albicans
[7].
According to the mechanism of action, Uro-vaxom is an immunomodulator; Experimental studies have established its activating effect on lymphocytes and macrophages. Uro-vaxom increases both humoral and cellular immune responses, thereby enhancing natural defenses against microbial agents. Uro-vaxom is initially prescribed in combination with antimicrobial therapy; the main goal is to reduce the frequency and severity of relapses and exacerbations. In the future, it is possible to carry out preventive courses of Uro-Vaxom. Unusually important results were obtained by Bottex et al. [5], who found that Uro-vaxom significantly reduces immunosuppression caused by antibiotics
.
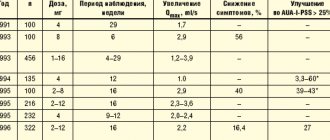
Foreign experience shows the feasibility of using Uro-vaxom in clinical practice. Schulman et al. [13] assessed the effectiveness of Uro-Vaxom on the example of 160 patients with urinary tract infections. 82 of them received Uro-Vaxom (mean age 45.3±2.0 years, 84% women) and 78 received placebo (age 45.0±1.8 years, 83% women). Patients received Uro-vaxom 6 mg or placebo once a day for 3 months; Then they were monitored for another 3 months. Bacteriuria was recorded significantly less frequently in the Uro-Vaxoma group than in the control: 31 versus 59 during treatment (p<0.001) and 27 versus 55 during the observation period (p<0.001). In the Uro-Vaxom group, 0.7 cases of relapse were noted over 6 months, while in the control this occurred 2 times more often. Accordingly, patients in the Uro-Vaxom group took antibiotics significantly less. The authors emphasize the long-term aftereffect in the main group. Side effects in the form of minor skin manifestations were noted in 2 patients receiving Uro-Vaxom and in 11 patients receiving placebo. Magasi et al. [10], who conducted a study using the same design with 112 patients, obtained similar data: in patients treated with Uro-Vaxom, exacerbation was observed in 13.8% of cases within six months, while among those taking placebo - in 79.6 %. These authors did not record any adverse reactions at all. The effectiveness of Uro-Vaxom was studied on the example of 64 patients with spinal trauma (67% of them were men). All of them underwent regular or continuous bladder catheterization; All of them had an increased number of leukocytes in their urine. In most patients, growth of E. Coli
;
in isolated cases – growth of Proteus spp., Klebsiella spp;
S. aureus .
Patients were given a three-month course of antibacterial therapy followed by Uro-Vaxom or placebo (also for 3 months). In both groups, while taking antibiotics, a significant improvement was obtained, which persisted in patients receiving Uro-Vaxom, but returned to the original level in the placebo group. Minor side effects were noted by 6 and 5 patients, respectively [6]. The long-term aftereffect of Uro-Vaxom was noted by Tammen et al. [15]. In addition to the standard regimen (3 months of Uro-Vaxom, 3 months of observation), the authors continued to monitor the patients’ condition for another 5 months. The main group included 61 patients (85% women), and the control group receiving placebo included 59 patients (86% of them women). Over the course of six months, patients in the control group had an average of 1.8 exacerbations of urinary tract infections, while in the main group this figure was only 0.82 (p <0.05). Although antibiotic therapy in the first 3 weeks of the study was similar in both groups, subsequently the need for repeated courses of antibiotics in the main group was significantly lower. Clinical manifestations of urinary infections
(pyuria, bacteriuria, dysuria)
stopped faster when taking Uro-Vaxom
.
Overall, the effectiveness of complex treatment in the main group was 95.1%, while in the control group it was only 72.9%. The positive effect persisted throughout the observation period in 95.1% of patients receiving Uro-Vaxom and in 60.3% of patients in the placebo group. A method of fractional therapy is also described (the first 10 days of each month for three months in a row). Thus, Schneider [12] prescribed Uro-Vax to 116 women (average age 35.6 years) suffering from uncomplicated cystitis. About a quarter of the patients were included in the study during the period of remission. Treatment was carried out for 10 days every month for six months. Previously, these patients annually noted an average of 3.4 exacerbations of cystitis; against the background of intermittent use of Uro-Vaxom, their number decreased to 1.2. This treatment regimen was also well tolerated by patients, the severity and frequency of side effects were insignificant. The feasibility of maintenance therapy with a booster dose of Uro-Vaxom in postmenopausal women has been proven. Uro-vax was prescribed to 58 women (average age 66.3 years), who had an average of 3.4 exacerbations of chronic cystitis annually. The treatment regimen was as follows: for 3 months, patients took Uro-Vaxom, 1 capsule daily, followed by a three-month break, then for another 3 months, patients received Uro-Vaxom 10 days a month. In just 9 months of observation, the relapse rate decreased by 64.7%, averaging 1.8. A slight heaviness in the stomach after taking the drug was noted in one patient; no other complications of treatment were recorded [11]. There is another aspect to the problem of treating urinary infections. In the Ural, Siberian and Far Eastern federal districts (74% of the territory of Russia), a tuberculosis epidemic persists, that is, the incidence of the population exceeds 100 per 100,000 [4]. In these conditions, recommendations for the treatment of infections of the genitourinary system, created for safe conditions, cannot be fully applied, since there is a risk of reducing the inoculation of Mycobacterium tuberculosis (MBT) and, consequently, late diagnosis of urotuberculosis, and the frequency of drug resistance of MTB increases. In a tuberculosis epidemic zone, treatment of supposedly nonspecific infections should begin with drugs that obviously do not have an inhibitory effect on Mycobacterium tuberculosis: Amoxicillin/clavulonic acid
is a broad-spectrum antibiotic that has a bactericidal effect, including against strains resistant to amoxicillin.
Gentamicin
is a broad-spectrum aminoglycoside.
Bactericidal, penetrates the cell membrane. Fosfomycin trometamol
is a broad-spectrum antibiotic that is active against most gram-u1086 negative and gram-positive bacteria.
After a single oral dose of fosfomycin trometamol, a concentration is created in the urine after 3 hours that is significantly higher than the minimum inhibitory concentration for the main uropathogenic strains of bacteria, and persists for 72–80 hours. Currently, the lowest microflora resistance has been registered to fosfomycin trometamol [9]. Ceftriaxone
is a third generation cephalosporin antibiotic that has a bactericidal effect.
Highly resistant to action? –lactamase. Effective against aerobic gram-positive and gram-negative microorganisms, including those resistant to penicillins and other cephalosporins. Excreted by the kidneys (60%) and through the intestines. Bactericidal concentrations persist for 24 hours. Nitrofurantoin
is an antimicrobial drug from the group of nitrofuran derivatives;
Available in the form of enteric-coated tablets. It has a bacteriostatic and bactericidal effect on gram-positive and gram-negative microorganisms, including those resistant to antibiotics and sulfonamides. Pyobacteriophage (sextaphage)
is a sterile mixture of purified filtrates of phagolysates of bacteria staphylococci, streptococci, Proteus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli;
is available ready-to-drink in the form of a transparent yellow liquid of varying degrees of intensity with a greenish tint. Uro-vaxom
should be considered as a drug that enhances the effectiveness of antibacterial therapy, and in some cases replaces it .
The therapeutic effect of Uro-Vaxom is due to stimulation of T-cell immunity, increased production of endogenous interferon and the creation of high levels of IgA in the urine [15]. Prescribed on an empty stomach, 1 capsule per day for 10 days for acute cystitis and up to 3 months for chronic urinary tract infections. Considering that the main causative agent of infectious and inflammatory diseases of the urinary tract is E. Coli
, the use of a specifically targeted drug that is not an antibiotic is very promising.
It is obvious that treatment with Uro-Vaxom will in no way affect the frequency of detection of MBT and will not cause the development of its resistance, therefore Uro-Vaxom is absolutely indicated for patients receiving ex juvantibus type 1 therapy [1]. The drugs listed above have a bactericidal effect on the main types of nonspecific microorganisms, without at the same time inhibiting the growth of Mycobacterium tuberculosis and, therefore, do not have a negative effect on bacteriological diagnostics. After performing 2-3 urine cultures for MBT, if there is no effect, fluoroquinolones (for example, norfloxacin or ciprofloxacin) can be prescribed. We used Uro-Vaxom in the complex treatment of 18 patients with recurrent infections of the genitourinary system. 15 had chronic nonspecific pyelonephritis, 2 had chronic cystitis and 1 had chronic urethritis supported by capitate urethral hypospadias. In 16 cases the causative agent was E. Coli
, in 1 –
Enterobacter
, in another patient –
S. aureus
.
Uro-vaxom was prescribed for a course of 3 months immediately after completion of antibacterial therapy. In no case were any adverse reactions to Uro-Vaxom noted. Patients came for a follow-up appointment, which included a general urine test, 2 times a month. The total observation period is 6 months. All patients achieved a stable effect, expressed in the absence of clinical manifestations of urinary tract infections, urine sanitization, and cessation of bacteriuria. None of the patients experienced an exacerbation of the disease. Thus, Uro-vaxom
should be considered as
a promising drug with etiopathogenetic action
for the treatment of urinary tract infections, which should be included in a complex therapy regimen. It is necessary to conduct a series of studies to determine the possibility of using Uro-Vaxom in the treatment of various categories of patients, including children, and, probably, expanding the indications.
Literature 1. Kulchavenya E.V. Difficulties in diagnosing tuberculosis of the genitourinary system. – Novosibirsk, 2004. 2. Rafalsky V.V., Strachunsky L.S., Krechikova O.I. et al. Resistance of pathogens of outpatient urinary tract infections according to multicenter microbiological studies UTIAP-I and UTIAP-II.//Urology.–2004.–No. 2.– P. 14–17. 3. Rafalsky V.V., Strachunsky L.S., Kogan M.I. et al. Antibacterial therapy of complicated urinary tract infections in outpatients.//Urology. – 2004. – No. 5. – P. 25–29. 4. The state of anti-tuberculosis care to the population of the Siberian and Far Eastern federal districts based on the results of work in 2003 / ed. Krasnova V.A. – Novosibirsk, 2004. 5. Bottex C., Martin A., Fontangens R. Action of a mycotoxin on the immune response of the mouse – interaction with an immunomodulator. Immunopharm Immunotoxic 1990; 12: 311–325. 6. Hachen HJ Oral immunotherapy in paraplegic patients with chronic urinary tract infections: a double-blind, placebo-controlled trial. // J. Urol., 1990; 143:759–763. 7. Hockertz St. Immunomodulatory effects of immunoactive fractions of selected E.Coli strains on the macrophage. Arzneim Forsch Drug Res. 1990; 40: 1068–1072/ 8. Johnson JR, Stamm WE. Urinary tract infections in women: diagnosis and treatment. Ann Intern Med 1989; 111:906–917. 9. Kahlmeter G., Journal of Antimicrobial Chemotherapy (2000) 46 Suppl., S1 – 15 –22 (cited from the journal “Medical Estate”, 2004. – No. 3. – pp. 32–36. 10. Magasi P, Panovics J, Illes A, Nagy M. Uro–Vaxom and the management of recurrent urinary tract infection in adults: a randomizes multicenter double–blind trial. Europ Urol 1994;26:137–140. 11. Popa G, Lauber K–D , Rothe H, Rugendorff E. Rezidivierende Harnwegsinfektionen in der Postmenopause. Wirksamkeit einer oralen Immuntherapie mit E. Coli-fraktionen. Munch med Wschr 1996; 138: 713–716. 12. Schneider H–J. New therapeutic approach for recurrent urinary tract infections Marked reduction in recurrence rate in women with uncomplicated cystitis – few side effects, high compliance. Der Allgemeinarzt 1990;12:626–633. 13. Schulman CC, Corbusier A, Michiels H, Taenzer HJ. Oral immunotherapy of recurrent urinary tract infections : a double-blind placebo-controlled multicenter study J Urol 1993;150:917–921 14. Schultz HJ, McCaffrey LA, Keys TF, Nobrega FT. Acute cystitis: a prospective study of laboratory tests and duration of therapy. Mayo Clin Proc 1984; 59: 391–397. 15. Tammen H. and the German urinary tract infection study group. Immunobiotherapy with Uro-Vaxom in recurrent urinary tract infection. //Br J Urol 1990; 65:6–9.
Uro-Vaxom®
Activation of the immune response after oral administration of the drug Uro-Vaxom® begins in the area of Peyer's patches of the small intestine. Strengthening the immune response in the genitourinary tract under the influence of the drug Uro-Vaxom® occurs in two directions:
1. Activation of the humoral immune response (activation of T- and B-lymphocytes, synthesis of various anti-E. coli antibodies, synthesis of class A immunoglobulin).
2. Activation of a nonspecific immune response (macrophages and NK cell phagocytosis).
Thanks to this activation, Uro-Vaxom® is effective against not only E. coli, but also against other uropathogens, including various serotypes of E. coli and/or pathogens belonging to other bacterial genera.
In vitro studies have shown that Uro-Vaxom® stimulates the activity of macrophages and neutrophils, activates the maturation of dendritic cells and increases the expression of adhesion molecules by neutrophils. The activation and maturation of dendritic cells are central to the cellular response to trigger an appropriate immune response in the intestine.
Due to the activation of B lymphocytes, the synthesis of immunoglobulin A increases, incl. in urine. In addition, studies on mice have shown that Uro-Vaxom® increases the activity of leukocytes in the blood and the secretion of tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6), interleukin-12 (IL-12), interferon -alpha (IFN-alpha) by peritoneal cells, as well as immunoglobulin G (IgG) in spleen cell culture. The molecular mechanism by which Uro-Vaxom® stimulates innate immune cells is likely related to its ability to activate pattern recognition receptors (PRRs). In vitro studies using HEK.293 cell lines with the expression of membrane and cytoplasmic pattern recognition receptors (Toll-like receptors (TLRs) or Nod-like receptors (Nod)) and the presence of reporter genes, it was found that the components contained in the drug Uro-Vaxom® are recognized by receptors TLR2 and TLR4 and to a lesser extent Nod2, but are not recognized by receptors such as TLR3, TLR5, TLR7, TLR8 or TLR9. Activation of these receptors, associated with pathogen-associated molecular patterns (PAMPs), including small molecules of the bacterial wall from the drug Uro-Vaxom®, triggers a cascade of immune reactions.
The drug Uro-Vaxom® promotes the following effects:
— stimulates T and B lymphocytes;
— stimulates the activity of dendritic cells, macrophages, NK cells and neutrophils;
- induces the formation of endogenous interferon (IFN), tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6), interleukin-12 (IL-12);
— increases the content of immunoglobulin A (IgA), including in the urine.
The drug Uro-Vaxom® has an immunostimulating effect and activates B lymphocytes (production of polyclonal antibodies), macrophages (impact on the function of phagocytosis) and dendritic cells (activation of maturation markers).
When taking the drug Uro-Vaxom®, the frequency of relapses of urinary tract infections, especially cystitis, decreases.