What drugs are used in oncology
The drugs are included in international schemes and protocols for the treatment of prostate cancer, which are used by oncology specialists.
In particular, the most common combinations are docetaxel + prednisolone, cabazitaxel + prednisolone. Medicines have different mechanisms for destroying malignant cells. For example, docetaxel acts on the cell membrane, making it elastic, which is why the cell loses its ability to divide and is destroyed.
Modern drugs have a low toxic effect on healthy tissue and quickly destroy cancer cells. The degree of toxicity and the severity of side effects of chemotherapy in a patient depend on the individual selection of drugs, their dosage, as well as the characteristics of the body.
Chemotherapy for prostate cancer: how it is treated
The Oncology Center has a full range of diagnostic equipment for prostate cancer research. Each patient is examined using high-precision digital equipment and undergoes diagnostics in the oncology center’s own laboratory.
The attending physician analyzes examination data, assesses the patient's health status, identifies possible contraindications, and draws up an individual treatment plan in accordance with accepted protocols and regimens.
The number of chemotherapy courses for this disease is determined by the attending physician depending on international clinical recommendations. The length of treatment for prostate cancer depends on how effectively the medications work and how the patient tolerates them. Several courses with breaks of 1-4 weeks can last up to six months.
Proven treatment regimens, precisely selected drugs and doses reduce the burden on the body and reduce the negative effects of chemotherapy, alleviating the condition of patients. After completion of therapy, the oncology department offers rehabilitation programs to restore the body.
Chemotherapy for prostate cancer (prostate): treatment effect
To determine how effective the therapy is, the patient undergoes regular studies. In medicine, there are 4 assessments of the effect of chemotherapy:
- Full answer . The neoplasm and metastases completely disappear, and this condition is maintained for at least 4 weeks from the moment of documentation of the disappearance of tumor lesions.
- Partial answer . In 2 studies, a reduction in cancer lesions of 50 percent or more was observed within 4 weeks, and the disease itself did not progress.
- Stabilization . There is no progress in the development of the disease, but the study shows inconsistency with the criteria for complete or partial response.
- Progression . The tumor continues to grow, new foci and metastases appear.
No matter how the disease develops, the doctors at the oncology center will make every effort to reduce the severity of symptoms and increase the patient’s life expectancy.
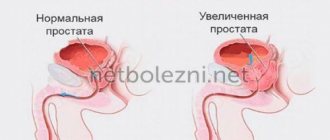
For prostate adenoma
Home Medical Encyclopedia Medicines Medicines used for prostate adenoma
See also sinestrol, speman, testosterone propionate, “tetrasterone” solution in oil for injection.
ADENOPROSTAL (Adenoprostal)
Plant pollen extract.
Pharmachologic effect. Stimulates metabolism and has anti-inflammatory properties.
Indications for use. Prostatitis (inflammation of the prostate gland), adenoma (benign tumor) of the prostate gland, frequent urination.
Method of administration and dose. 1 capsule 2 times a day for the first week. Then 1 capsule per day under the supervision of a physician.
Release form. Capsules in a package of 10 pieces.
Storage conditions. In a well-packed container, protected from light and moisture.
ADENOSTOP (Adenostop)
Pharmachologic effect. Adenostop is a liquid extract of the herb Cocklebur prickly. Due to the presence of flavonoids, essential oils, phytosterols, and tannins in the extract, the drug has a decongestant and antimicrobial effect. When using the drug, reverse development of the hypertrophied (increased in volume) prostate gland is observed. The drug significantly retains potassium and magnesium ions in the body.
Indications for use. Prostate hypertrophy (for patients who cannot undergo adenomectomy - surgical removal of a benign prostate tumor); auxiliary treatment before and after adenomectomy; cystopyelitis (combined inflammation of the renal pelvis and bladder), urinary incontinence.
Method of administration and dose. Dilute 40-45 drops in 30 ml of water. Take 2 times a day 30 minutes before meals, you can follow with a glass of water.
Contraindications. Insufficiency of adrenal function.
Release form. Concentrate for the preparation of a solution for oral administration in a bottle of 85 ml.
Storage conditions. In a dry, cool place, protected from light.
AMNIOCENE (Amniocenum)
Denatured amniotic membrane of the human placenta (destroyed amniotic membrane).
Pharmachologic effect. A biogenic stimulant that has an anti-inflammatory and absorbable effect.
Indications for use. Chronic prostatitis (inflammation of the prostate gland), adenoma (benign tumor) of the prostate of the I and II degrees, as well as prostate adenoma in patients who are not subject to surgical treatment. Sometimes used in gynecological practice for chronic salpingoophoritis (inflammation of the uterine appendages) and parametritis (inflammation of the periuterine spaces).
Method of administration and dose. Amniocene is injected under the skin along the mid-axillary line at the level of the VII-VIII ribs through a thick long needle, 5 ml once every 5-7 days. In case of poor tolerability of the drug, 2 ml is administered at this dose, followed by increasing the volume of each injection by 1 ml, bringing it to 5 ml. Before injection, the bottle with the drug is heated to body temperature and shaken. The course of treatment consists of 6-8 injections.
The patient should be warned about the possibility of a local reaction and an increase in body temperature.
Side effect. The use of amniocene is accompanied by a local reaction in the form of pain, swelling, and in some cases the formation of long-term non-absorbable infiltrates (indurations) is possible. At the beginning of treatment, an increase in body temperature, headache, and general weakness are often observed, which do not require discontinuation of the drug. During the day after administration, a short-term increase in ESR (erythrocyte sedimentation rate), slight leukocytosis (increase in the number of leukocytes in the blood), eosinophilia (increase in the number of eosinophils in the blood) are possible. The general reaction occurs, as a rule, among young people. In case of a significant increase in body temperature (above 38 ° C), conventional antipyretic drugs are prescribed. Some patients may experience an allergic reaction in the form of a skin rash. In these cases, the drug should be discontinued.
Contraindications. Amniocene is contraindicated in cases of severe hypertension (persistent increase in blood pressure), cardiovascular failure, tumors, purulent processes in the uterus, appendages, abscesses (ulcers) in the abdominal cavity, intolerance to protein drugs.
Release form. Suspension (suspension of solid particles in liquid) in 5 ml bottles, 10 bottles in a package.
Storage conditions. In a dark place at a temperature from +6 to +10 °C.
Gestonorone caproat
Synonyms: Depostat, Gestronolhexanoate, Primostat, etc.
Pharmachologic effect. In case of adenoma (benign tumor) of the prostate, the drug at the cellular level inhibits the stimulating effect of the metabolite of testosterone - the male sex hormone.
Indications for use. Prostate adenoma, mainly in patients with contraindications to surgical treatment.
Method of administration and dose. Administer 200 mg intramuscularly once a week. A 2-3 month course is recommended. Before administration, the solution in the ampoule is heated to body temperature.
Side effect. Immediately after administration, short-term shortness of breath is possible. With prolonged use, gynecomastia (enlarged mammary glands in men) and impaired potency are possible.
Contraindications. The drug should be used with caution in patients with liver disease, kidney disease, diabetes mellitus, bronchial asthma, epilepsy, and migraine.
Release form. 10% oil solution in ampoules of 2 ml, in a package of 5 pieces. 1 ml of solution contains 100 mg of the drug.
Storage conditions. In a place protected from light at a temperature not exceeding +25 °C.
PEPONEN (Reropep)
Pharmachologic effect. The drug is a natural pumpkin seed oil, which contains sterol, squalene, unsaturated fatty acids, vitamin E, coenzyme Q, and selenium. Peponen relieves (relieves) dysuric symptoms (disorder of urination) observed with adenoma (benign tumor) of the prostate gland. Relieves pain in patients with prostatitis (inflammation of the prostate gland). Helps increase potency in men. The drug activates the body's immune (defense) systems. With long-term use, peponen has a hypolipidemic effect (reduces lipid levels in the blood).
Indications for use. Initial stage of prostate adenoma, hyperlipidemia (increased fat content in the blood) On and (16 types, prevention of atherosclerosis.
Method of administration and dose. For prostate adenoma, 1-2 capsules are prescribed 3 times a day. For hyperlipidemia, 1-2 capsules are prescribed 3 times a day. The drug can be used either as monotherapy (treatment with peponene alone) or in combination with other lipid-lowering agents. To prevent atherosclerosis, peponen is prescribed 1-2 capsules 2-3 times a day. Therapy is carried out for a long time.
Contraindications. Hypersensitivity to the drug.
Release form. Capsules containing 0.3 g of pumpkin seed oil, in a package of 100 pieces.
Storage conditions. In a dry place, protected from light.
PRAZOSIN (Prazosinum)
Synonyms: Adverzuten, Deprazolin, Minipress, Pratsiol, Decliten, Duramipress, Eurex, Furazosin hydrochloride, Hypovaz, Orbizan, Patsolin, Peripress, Prazak, Prazopress, Sinetens, Vasoflex, etc.
Pharmachologic effect. Selective alpha-blocker. The effect of the drug in adenoma (benign tumor) of the prostate is based on the fact that urinary disorders in patients are largely associated with increased excitability of alpha-adrenergic receptors of the bladder, and blockade of alpha-adrenergic receptors of the bladder neck, posterior urethra (back of the urethra) and prostate glands improves the dynamics of urination.
Indications for use. Prostate adenoma.
Method of administration and dose. For prostate adenoma, prazosin is prescribed orally regardless
write from reception. The first dose, equal to 0.5 mg, is taken before bedtime. After taking the first dose, the patient should stay in bed for at least 6-8 hours, since the first dose of the drug may be accompanied by a sharp drop in blood pressure. This reaction is especially pronounced against the background of previous use of diuretics. In this regard, the dose of the drug should be selected with extreme caution in elderly people. Next, 1 mg is prescribed 2 times a day for a week. Then increase the dose to 4 mg per day (1 mg 4 times a day). If a pronounced hypotensive reaction occurs (severe decrease in blood pressure), the daily dose can be reduced to 2 mg. The course of treatment is 8 weeks, can be extended if necessary. After stopping the main course of treatment, patients can be prescribed a maintenance dose of 2 mg per day (1 mg 2 times a day).
Side effect. A sharp drop in blood pressure, dizziness, headache, insomnia, weakness, fatigue, nausea, diarrhea, constipation, dry mouth. Usually these phenomena go away on their own. In some cases, increased heart rate may occur.
Contraindications. For patients with kidney damage, the drug is prescribed in reduced doses with caution.
Release form. Tablets of 0.001 and 0.005 g (1 and 5 mg) with a score in a package of 50 or 100 pieces.
Storage conditions. List B. In a place protected from light.
PROSCAR
Synonyms: Finasteride.
Pharmachologic effect. Inhibits (suppresses activity) alpha reductase (a protein enzyme that accelerates chemical reactions in the body), which converts testosterone (male sex hormone) into more active dehydrotestosterone, reduces its content in the blood and prostate tissue. Inhibits the stimulating effect of testosterone on the development of adenoma (benign tumor) of the prostate gland. With long-term use, it causes a decrease in the size of the prostate gland and helps normalize urination.
Indications for use. Prostate adenoma.
Method of administration and dose. Take 5 mg of the drug once a day. The effectiveness of the therapy can be assessed after at least 6 months. after starting treatment. In patients with a large volume of residual urine and/or sharply reduced urination, careful monitoring should be carried out for the possible development of obstructive uropathy (damage to the urinary organs due to difficulty in the outflow of urine).
Side effect. In rare cases, impotence (sexual impotence), decreased libido (sexual desire), decreased volume of ejaculate (seminal fluid), and gynecomastia (enlarged mammary glands in men) are possible.
Contraindications. Hypersensitivity to the drug.
Release form. Tablets of 0.005 g in a package of 28 pieces.
Storage conditions. In a dry place, protected from light.
PROSTATILEN (Prostatilene)
It is a complex of water-soluble peptides isolated from the prostate gland of cattle.
Pharmachologic effect. Strengthens the vascular wall, helping to restore microcirculation. The administration of the drug increases the functional activity of smooth muscle cells of the bladder and prostate gland, and also has an activating effect on the state of the immune system.
Indications for use. Used for chronic prostatitis (inflammation of the prostate gland), adenoma (benign tumor) of the prostate gland, reflex urinary retention.
Method of administration and dose. Apply intramuscularly at 5-10 mg daily once for 5-10 days. The contents of the bottle are dissolved immediately before use in 1-2 ml of isotonic sodium chloride solution, water for injection or 0.5% novocaine solution. Repeated courses of treatment are recommended no earlier than after 2-4 weeks.
Side effect. Not found.
Contraindications. Individual intolerance to the drug.
Release form. Lyophilized (vacuum-freeze-dried) powder in 5 mg vials.
Storage conditions. In a place protected from light at a temperature not exceeding +20 °C.
RAVERON
Extract of the prostate gland of sexually mature animals, freed from androgens (male sex hormones) and estrogenic hormones (female sex hormones) and proteins.
Pharmachologic effect. Biogenic stimulant. When using the drug in patients with prostate adenoma (benign tumor) and chronic prostatitis (inflammation of the prostate gland), the frequency of urination usually decreases and bladder emptying improves.
Indications for use. Used in the initial phase of prostate adenoma and chronic nonspecific prostatitis. The use of the drug does not replace surgical intervention. It is recommended mainly in the presence of contraindications to surgery, as well as in postoperative urinary incontinence.
Method of administration and dose. The drug is administered deeply intramuscularly: on the first day 0.3 ml, on the second day 0.5 ml and then daily 1 ml per day or 2 ml every other day for 4-6 weeks. Courses of treatment can be repeated, again starting from 0.3-0.5 ml.
Side effect. When using the drug, allergic reactions (skin rash, etc.) are possible; in these cases, the injections are stopped.
Contraindications. Tendency to allergic reactions.
Release form. In ampoules of 1 ml in a package of 10; 30 or 50 pieces.
Storage conditions. In a cool place.
SERPENCE
Synonyms: Permixon.
Lipid stearic extract from the Serenoarepens plant.
Pharmachologic effect. Slows down the development of prostate adenoma (benign tumor) and reduces the symptoms of the disease. The mechanism of action of the drug is mainly associated with inhibition (suppression of activity) of the enzyme 5-alpha reductase, which ensures the transformation of testosterone into dihydrotestosterone, and thereby prevents the proliferation of prostate tissue. In addition, the drug has an anti-edematous effect.
When taken over a long period of time, the drug can effectively control the development of the disease and prevent the occurrence of complications, and therefore the need for surgical treatment.
Does not affect sexual activity.
Indications for use. Functional disorders associated with hypertrophy (increase in volume) of the prostate. The drug is especially indicated in the treatment of benign prostatic hypertrophy and associated symptoms: frequent urination both during the day and at night, with a decrease in the volume and force of urination, accompanied by a painful sensation of tension in the perineum when the bladder is completely emptied.
Method of administration and dose. 160 mg 2 times a day (morning and evening) with meals. Treatment is recommended over a long period of at least 30 days.
Side effect. In rare cases, nausea may occur, especially when taking the drug on an empty stomach.
Contraindications. Increased individual sensitivity to the drug.
Release form. Film-coated tablets, 0.08 g (80 mg), in a package of 60 pieces; soft capsules of 0.16 g (160 mg), in a package of 30 pieces.
Storage conditions. List B. In a place protected from light.
TRIANOL
African plant bark extract - Pugeutafricanum.
Pharmachologic effect. Biogenic stimulant. The drug helps to facilitate urination in diseases of the prostate gland, including adenoma (benign tumor); a decrease in inflammatory and dysuric phenomena (urinary disorders) and an improvement in the secretory activity of the prostate gland are observed.
Indications for use. Used for prostate adenoma and chronic nonspecific prostatitis (inflammation of the prostate gland). The use of the drug does not replace surgical intervention.
Method of administration and dose. Take 2 capsules 2 times a day before meals. The course of treatment is carried out for 4-6 weeks. and repeat after 4-6-8 months.
Side effect. In some cases, dyspeptic symptoms (digestive disorders), mild allergic reactions, pain in the testicles, and gynecomastia (enlarged mammary glands in men) are possible.
Release form. Capsules containing 25 mg of trianol, in a package of 30 pieces.
Storage conditions. In a cool, dark place.
TYKVEOL (Tycveolum)
A complex of biologically active substances obtained from pumpkin (carotenoids, tocopherols, phospholipids, sterols, phosphatides, flavonoids, vitamins Bi, 62, C, P, PP, F, saturated, unsaturated and polyunsaturated fatty acids - palmitic, stearic, oleic, linoleic, linolenic, arachidic).
Pharmachologic effect. Reduces proliferation (increase in number) of prostate cells. It also has hepatoprotective (protects the liver), choleretic, antiulcer, antiseptic (disinfecting) and antisclerotic effects.
Indications for use. Prostatitis (inflammation of the prostate gland), adenoma (benign tumor) of the prostate gland; hepatitis (inflammation of liver tissue), fatty liver (liver disease associated with impaired fat metabolism), cirrhosis of the liver, cholecystocholangitis (combined inflammation of the gallbladder and bile ducts), dyskinesia (impaired mobility) of the biliary tract, gastritis (inflammation of the stomach), ulcerative disease of the stomach and duodenum, heartburn, colitis (inflammation of the colon), enterocolitis (inflammation of the small intestine), hemorrhoids (bulging and inflammation of the veins of the rectum); atherosclerosis; erosion (superficial defect of the mucous membrane) of the cervix. Externally for herpes (a viral disease characterized by a rash of grouped blisters on the skin and/or mucous membranes), dermatitis (inflammation of the skin), diathesis, psoriasis, eczema; burns and burn disease; periodontal disease (inflammation of the tissues surrounding the tooth root).
Method of administration and dose. Orally 30 minutes before meals, 1 teaspoon 3-4 times a day for 3-4 weeks. Apply topically to the affected areas 2-3 times a day. For prostatitis and hemorrhoids, along with oral administration, it is used rectally (into the rectum) in the form of microenemas. In gynecology, it is used in the form of vaginal tampons 2 times a day. For periodontal disease - applications (physiotherapeutic procedure) of pumpkin.
Side effect. Dyspepsia (digestive disorders), diarrhea (diarrhea).
Contraindications. Hypersensitivity to the drug.
Release form. Available in bottles of 50 and 100 ml. Three teaspoons of the drug contain a daily dose of vitamins A, E, F, B.
Storage conditions. In a place protected from light at a temperature of +10 to +30 °C.
URTIRON
Pharmachologic effect. A combined preparation containing nettle root extract and betasitosterol. It influences metabolic (metabolic) processes, inhibits the progression of prostate enlargement and prevents inflammation in the gland.
Indications for use. Non-infectious chronic prostatitis (inflammation of the prostate gland), recovery period after surgery for adenoma (benign tumor) of the prostate gland. Difficulty urinating caused by adenoma or hypertrophy (increase in volume) of the prostate gland at the initial stage of the disease.
Method of administration and dose. At the beginning of treatment, 1 capsule is prescribed 3 times a day. After relief occurs, the dose is reduced to 1 capsule 2 times a day. The duration of treatment is 6-8 weeks. If necessary, the course of treatment can be repeated after 6 months.
Side effect. Allergic reactions are possible.
Contraindications. Hypersensitivity to the components of the drug.
Release form. Capsules containing 300 mg of nettle root extract and 10 mg of betasitosterol, in a package of 40 pieces.
Storage conditions. In a dry place.
Cernilton
Tablets containing plant pollen extract.
Pharmachologic effect. Biogenic stimulant with anti-inflammatory and metabolism-stimulating effects.
Indications for use. Chronic prostatitis (inflammation of the prostate gland), adenoma (benign tumor) of the prostate gland.
Method of administration and dose. Start taking 6 tablets (once) per day immediately after breakfast. After a few days, reduce the dose to 3-4 tablets (once). The drug is taken for a long time.
Release form. Tablets of 0.4 g in bottles of 100, 250 and 1000 pieces.
Storage conditions. In a dry place, protected from light.
| print version | This information is not a guide to self-treatment. A doctor's consultation is required. |
Contraindications
Chemotherapy for prostate cancer is contraindicated in patients:
- with hypersensitivity to a particular drug;
- severe dysfunction of the liver and kidneys;
- severe concomitant diseases;
- significant depletion of the body.
Oncology doctors determine the presence of contraindications for each patient and adjust the treatment program taking them into account. A professional approach to planning and conducting treatment allows you to achieve lasting positive results.
SUPPOSITORIES WITH PUMPKIN SEED OIL in the treatment of chronic nonspecific prostatitis
Nonspecific inflammation of the prostate gland is the most common urological disease in men of working and reproductive age. According to statistics, chronic nonspecific prostatitis occurs in 35–40% of men, mainly at the age of 20–40 years (Yunda I.F., 1987).
In the pathogenesis of the disease, the microbial factor plays a significant role only in the initial phase of its development, later it plays a secondary role, and the pathological changes that arise in the tissues of the prostate gland, stagnation of secretions, neurotrophic disorders, etc., become of primary importance.
Treatment of chronic nonspecific prostatitis is complex. Among the medicines used in the pharmacotherapy of this disease, herbal medicines, in particular those made from pumpkin seed oil, are of no small importance. The effect of these drugs is due to the biologically active substances (BAS) included in their composition, namely: fatty acids (palmitic, stearic, oleic, linoleic), sterols, phospholipids, carotenoids, vitamins and microelements.
The action of biologically active substances is manifested by the normalization of urination, the elimination of pain, the normalization of immune status, and the disappearance of sexual dysfunction.
Pumpkin seed oil is used in various dosage forms (capsules, bottles). For the treatment of nonspecific prostatitis, the more appropriate route of drug administration is rectal, since it ensures a more effective delivery of drugs into the prostate tissue.
SUPPOSITORIES WITH PUMPKIN SEED OIL were developed by Monfarm OJSC together with the State Scientific Center for Drugs and Drugs (Kharkov) and Monfarm OJSC began producing this drug. In the Department of Sexopathology and Andrology of the Institute of Urology and Nephrology of the Academy of Medical Sciences of Ukraine under the leadership of Professor I.I. Gorpinchenko, a comparative limited clinical study of the therapeutic effectiveness and tolerability of SUPPOSITORIES WITH PUMPKIN SEED OIL and an imported drug in capsule form was conducted.
The study included 60 patients (30 each in the main and control groups) diagnosed with chronic prostatitis.
The criteria for inclusion of patients in the study were:
- gender - male
- age - over 18 years old
- established diagnosis - chronic prostatitis
- obtaining informed written consent from the patient to participate in the study
- the patient’s ability to adequately cooperate during the study period.
The criteria for excluding patients from the study were:
- history of hypersensitivity to the drug
- the presence of diseases in the acute phase or acute diseases that can significantly affect the results of the study
- the patient’s inability to refuse alcohol and/or drugs during the study period
- participation in any other clinical trial.
Conditions for patient withdrawal from the study:
- development of severe and/or unexpected side effects during the study period
- significant deterioration in general condition during treatment
- non-compliance with the drug regimen
- refusal to participate in the study.
Patients were distributed into groups using random sampling.
Patients of the main (1st) group used SUPPOSITORIES WITH PUMPKIN SEED OIL rectally 2 times a day (1 g of active substance) daily during the entire study period.
Patients in the control (2nd) group took a comparison drug - capsules containing pumpkin seed oil, orally 3 times a day (0.9 g of active substance) after meals every day during the entire study period.
The patients of both groups did not differ in age and duration of the disease (Tables 1, 2).
Table 1
Patient age, years
| Index | Main group | Control group |
| Average value | 45,1 | 42,1 |
| Standard error | 2,1 | 2,2 |
| Minimum value | 19 | 23 |
| Maximum value | 73 | 63 |
table 2
Duration of disease, years
| Index | Main group | Control group |
| Average value | 5,9 | 5,2 |
| Standard error | 0,88 | 0,96 |
| Minimum value | 3 months | 3 months |
| Maximum value | 20 | 18 |
On transrectal palpation of the prostate gland, pain was noted in all patients of the main group, changes in the consistency of the gland were detected in 18 (60%) patients, heterogeneity in 16 (53.3%), and dilated painful seminal vesicles in 6 (20%). Similar data were obtained in patients in the control group.
All examined patients were diagnosed with chronic diffuse prostatitis. A bacteriological study of prostate secretions did not reveal growth of pathogenic microflora in 24 (80%) patients in the main group and in 26 (86.6%) in the control group. The remaining patients showed growth of opportunistic microorganisms: Staphylococcus spp. —
in 5,
Streptococcus faecalis -
in 3,
Streptococcus anhaemolyticus -
in 2.
The results of general clinical blood and urine tests both before and after treatment with the study drug and the comparison drug did not differ from normal values.
Ultrasound examination (US) revealed a statistically insignificant decrease in prostate volume in patients of the main and control groups. Regarding the volumetric flow rate of urination, no significant differences were noted before and after treatment.
The residual urine volume indicator, which was also determined by ultrasound, underwent much more significant changes after therapy (Table 3).
Table 3
Volume of residual urine according to ultrasound, cm3
| Index | Group of patients | |||
| main | control | |||
| before treatment | after treatment | before treatment | after treatment | |
| Average value | 44,4 | 13,8 | 45,6 | 20,7 |
| Standard error | 7,5 | 4,9 | 8,8 | 3,6 |
Note: p=0.0015 - significance of differences in indicators before and after treatment for patients in the main group; p=0.013 - control; p=0.27 - main and control groups after treatment.
The results of the data in the table. 3 indicate that patients in both groups showed a marked decrease in the volume of residual urine. Moreover, after the use of SUPPOSITORIES WITH PUMPKIN SEED OIL, the tendency towards normalization of this indicator was more pronounced, although statistically unreliable, than after treatment with the comparison drug.
In patients of both groups, the severity of pain as a result of treatment decreased very significantly and statistically significantly. The severity of pain after treatment with pumpkin oil preparations decreased on average from moderate to slight; pain was absent or slightly severe after treatment in 26 (86.6%) and 24 (80%) patients of the main and control groups, respectively. There were no statistical differences in indicators between groups.
A similar picture was observed regarding dysuric phenomena. However, in patients of the main group, the severity of urinary disorders decreased more significantly. Urinary disorders after a course of treatment with SUPPOSITORIES WITH PUMPKIN SEED OIL disappeared 2 times more often (40% of patients) than after using the comparison drug (20%).
There was no statistically significant reduction in the severity of sexual dysfunction after therapy. However, the frequency of adequate and spontaneous erections increased in patients in both groups. In addition, patients in the main group noted an increase in the duration of sexual intercourse.
The results of assessing the effectiveness of therapy on a 5-point scale are shown in table. 4.
Table 4
Evaluation of the effectiveness of Suppositories with pumpkin seed oil
| Efficiency | Main group | Control group | |||
| number of patients | percent | number of patients | percent | ||
| 5 points – significant improvement | 6 | 20 | 5 | 16,7 | |
| 4 points - improvement | 19 | 63,3 | 15 | 50 | |
| 3 points - no change | 5 | 16,7 | 8 | 26,7 | |
| 2 points – slight deterioration | 0 | 0 | 2 | 6,6 | |
| 1 point – significant deterioration | 0 | 0 | 0 | 0 | |
The results obtained indicate a fairly high therapeutic effectiveness of the drug under study. There were no cases of deterioration in the condition of patients in the main group. The condition of 83.3% of patients improved to one degree or another. At the same time, after using the comparison drug, similar results were achieved only in 66.7% of patients. The higher therapeutic effectiveness of SUPPOSITORIES WITH PUMPKIN SEED OIL, with other similar indicators, is due to the local nature of the use of the active component. This allows you to create a higher concentration of the drug directly in the affected organ than with systemic use.
During treatment with SUPPOSITORIES WITH PUMPKIN SEED OIL, no side effects were identified.
Based on the data obtained during the clinical study, the following conclusions can be drawn:
- SUPPOSITORIES WITH PUMPKIN SEED OIL - a fairly effective remedy in the treatment of chronic nonspecific prostatitis, especially accompanied by urinary disorders
- The study drug helps to significantly reduce the volume of residual urine due to the elimination of spasm and swelling of the prostate tissue
- the drug is well tolerated by patients and does not cause side effects.
The results obtained allow us to recommend SUPPOSITORIES WITH PUMPKIN SEED OIL for inclusion in the complex treatment of patients with chronic nonspecific prostatitis, accompanied by disorders of urination and sexual function.
Based on materials provided by Monfarm OJSC