The use of the drug "Afala" in the treatment of urological diseases
12.05.2013
4308
0
Benign prostatic hyperplasia (BPH) has been a pressing and complex problem among diseases of the genitourinary system in men for many years. Despite the common belief that the disease is limited to older men, BPH tends to fall below age barriers and is often found in men under 50 years of age.
The greatest manifestation of the deterioration of the condition in this disease is manifested in an increase in the volume of the prostate gland, a decrease in the maximum rate of decrease in urination, aggravation of certain symptoms, and the risk of developing acute urinary retention, which leads to the inevitable prescription of surgical treatment. But research shows that the most common reason patients see a urologist for BPH is a decrease in quality of life. It is the irritating factors in the presence of this disease, in the opinion of most patients, that are the cause of the main concern, since they significantly affect daily activity and worsen the quality of life in general.
Today, in the treatment of patients with prostate adenoma, many pharmacological drugs are used that affect the mechanisms of the occurrence and development of the disease. At the present stage of development of urological practice in general, the drug Afala, which belongs to a new group of drugs, is very popular. The active component of Afala are antibodies to prostate-specific antigen (PSA), which affects the proliferation of prostate cells. Prostate specific antigen, later named PSA, was discovered by isolating a glycoprotein from prostate extract in 1979. Subsequent studies showed that this antigen has antiangiogenic activity and is involved in the growth of prostate stromal cells. With the help of messengers, namely insulin-like growth factor, PSA regulates proliferative processes in the prostate gland, which can cause benign pancreatic hyperplasia.
In 2009, a team of scientists from the Department of Urology of the Altai Medical University (head of the department A.I. Neymark) conducted a study that made it possible to identify the main effect of the drug Afala on the course of the disease, and thereby determine the optimal doses of the drug in the treatment of prostate adenoma.
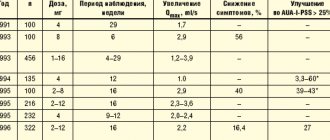
The study involved 145 patients aged 51 to 72 years, according to whose complaints and objective examination they were diagnosed with stage I - II BPH. The essence of this study was to analyze the main symptoms according to the IPSS and QoL scale, analyze the content of total PSA in the blood and determine the volume of the prostate gland, and also use traditional methods for diagnosing BPH. Study participants were divided into four groups: the first group included 31 patients who were to take Afala two tablets twice a day for a month; in the second group there were 30 people who took Afala two tablets four times a day for a month; the third group of subjects consisted of 39 patients who took the drug two tablets twice a day for 16 weeks, and the fourth group included 45 patients who took Afala two tablets three times a day for 16 weeks.
Patients were asked to answer the International Questionnaire for the Summary Assessment of Prostate Diseases and Quality of Life Assessment. According to the data obtained during this study, it turned out that in the first and second groups of patients, only one person did not notice either subjective or objective improvement due to taking the drug under outpatient supervision for a month, which is 1.7% of cases of the total number of patients . The remaining patients noted the absence of any adverse reactions, i.e. the drug was tolerated quite easily. In general, there was an improvement in the overall assessment of prostate diseases; there was a decrease in the symptoms of frequent daytime and night urination (more often than two hours later), including the absence of intermittent urination. In addition, there were no significant differences between these data in the two groups.
The study showed that there was also an improvement in the quality of life of patients. Thus, before taking the drug, among the patients of the first and second groups, almost half of the study subjects noted an unsatisfactory quality of life (48% of patients in each group), 17% of patients each noted the quality of life as poor, and in 34% it was observed as mixed. However, after 30 days of taking Afala, there was no unsatisfactory assessment of the quality of life in all those studied in the first and second groups. More than half of them noted a satisfactory quality of life (58.5 and 59%, respectively), two patients from each group rated the quality of life as excellent, the rest said they had mixed feelings.
The amount of residual urine in patients of the first and second groups was observed within limits close to the normative values, however, when statistically processing the data obtained before and after treatment, a significant decrease in this indicator was noted after treatment.
In general, the study of the first and second groups of patients showed that an improvement in the health status of patients with prostate adenoma is observed in the majority of patients during a 30-day course of taking the drug Afala.
The study of patients in the third and fourth groups was also carried out to evaluate the effectiveness using the IPSS and QoL questionnaires. Analysis of the data obtained in these groups shows that, in contrast to the first and second groups of patients, these groups of patients experienced a decrease in almost all IPSS indicators and a significant improvement in their overall health status.
Before taking this drug, 38% of patients noted their health status as unsatisfactory, 23% as poor, and 38% as mixed. After taking Afala, the following data were noted among the two study groups: the “unsatisfactory” rating was completely absent, 64% of patients described their health status as satisfactory, 30.7% as good, two patients indicated a mixed feeling.
In general, after treatment with Afala for 16 weeks, there was a decrease in the amount of residual urine, and PSA levels and prostate volume did not change.
In the fourth group of patients, before taking the drug, 50% of patients noted their quality of life as unsatisfactory, 27.7% as poor, and 22% as mixed. As in the third, in the fourth group of patients after taking the drug Afala there was no assessment of the quality of life as “unsatisfactory”; 67% of the subjects called it satisfactory, 16.7% - good, 11% - excellent and 5.5% noted a mixed feeling. The amount of residual urine in patients in this group also decreased significantly, and statistically insignificant changes in prostate volume and PSA levels were observed.
This study showed that the use of the drug Afala leads to a reduction in irritating urinary disorders in men suffering from BPH. In general, the effect of taking Afala is observed in 98% of cases, and the greatest effect is precisely in the first four weeks after taking it, and also increases with prolonged use. Increasing the dose of the drug to six tablets per day led to more pronounced changes in the quality of life assessment index. According to the data obtained from the above-mentioned studies, increasing the frequency of taking this drug up to three times a day most effectively eliminates weakening of the urine stream and nocturia.
Thus, the choice of dose of Afala for BPH depends on the severity of the symptoms of the disease. The drug can be recommended as a prophylactic agent for patients with the initial stage of prostate adenoma and as an alternative to expectant management. In general, Afala is safe for the health of patients; long-term use does not cause negative reactions.
The researchers argue that Afala should be recommended for wider use in the treatment and prevention of BPH.
Topics and tags
BPH
Afala
Comments
To post comments you must log in or register