A common pathology in men is prostate dysfunction. The disease is accompanied by unpleasant symptoms. The drug afolase belongs to a group of drugs whose action is aimed at treating benign prostate hypertrophy. The product is produced in the form of lozenges.
Features of the product
A common drug prescribed to prevent the development of prostate adenoma is afolase. The effectiveness of the drug has been confirmed in practice. This medicine has an affordable price. It can be bought at a pharmacy without a prescription.
The main components of the product are two types of antibodies. The instructions for use of the product describe in detail its pharmacological effects. When taken, a targeted medicine increases blood flow in the tissues of the prostate gland and penis. The active ingredients have a positive effect on endothelium, ensuring an improvement in the general condition of blood vessels. During treatment, functional and metabolic processes are normalized, inflammation and swelling are relieved.
The active substances included in the drug are affinity purified. That is, they undergo special processing to remove other proteins and components of the donor's DNA. This guarantees the absolute safety of the drug. When taking it, the risks of negative side reactions of the body are minimized.
Use of the drug "Afala" in the treatment of BPH
12.05.2013
5753
0
Today, benign prostatic hyperplasia is one of the most problematic diseases of the male genitourinary system. The negative quality of life of many men with prostatic hyperplasia is significantly influenced by the symptoms of the lower urinary tract. In this regard, modern drug therapy for this disease is represented by three groups of drugs: 5-alphareductase inhibitors (virtually do not affect the clinical symptoms of BPH during the first three to four months of treatment, but significantly improve the long-term prognosis of the disease), alpha-blockers (not influencing the tissue of the prostate gland, they effectively influence the dynamic component of obstruction, which contributes to the rapid relief of clinical symptoms of BPH: according to research, clinical symptoms improve by 20 - 50%, and urodynamic indicators - by 20 - 30%) and phytotherapeutic agents (the most studied is Serenoa repens extract).
Quite often, with such a disease, there is a need to use surgical treatment methods, since in a number of patients it is not possible to achieve the desired effect in the treatment of BPH, despite the high effectiveness of modern drugs. In addition, numerous side effects are also observed when taking them, which forces urologists to cancel the prescribed drugs.
The most appropriate treatment for BPH, according to most researchers, is first of all to restore normal PSA activity, since this substance regulates cell proliferation stimulated by insulin-like growth factor. With this disease, there is a fairly active reaction of the immune system to PSA - this is what led scientists to create the drug Afala, which is directly intended for the treatment of BPH, since thanks to the antibodies it contains to the prostate-specific antigen, it helps slow down the atrophic processes of the glandular epithelium and increases the zinc content in prostate tissue three times, which generally leads to the restoration of the functional activity of the prostate gland.
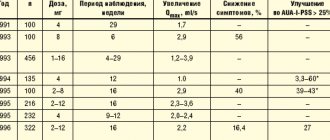
Two large studies have been conducted on the effect of Afala in the treatment of BPH. The first study of urologists from Rostov-on-Don (I.A. Aboyan, V.E. Aboyan, O.V. Zinkovskaya, V.A. Sknar, CDC “Health”) included 41 patients diagnosed with BPH, average age which ranged from 56 to 74 years. In general, when selecting study subjects, patients with the following criteria were excluded: age less than 45 and more than 75 years, symptoms of the disease are moderately severe, prostate volume was less than 25 ml, maximum urine flow rate was more than 15 ml/sec and less than 5 ml/sec. sec, as well as patients who have undergone surgery on the bladder or prostate gland in the past, patients with tumor diseases of the genitourinary system, with neurogenic disorders and with a urine volume of more than 150 ml.
The study included transrectal ultrasound examination of the prostate to determine organ volume, uroflowmetry to measure residual urine, PSA level determination, and a standard IPSS-QoL questionnaire.
All subjects took the drug Afala, two tablets twice a day for three months. At the first, third and seventh weeks of treatment, the patients' condition was assessed using the above questionnaire. Before the start of the study, basic data on the health status of the subjects were obtained: the average volume of the prostate gland in patients participating in the study ranged from 17.8 to 52.8 ml, the volume of residual urine was 14.5 - 30.6 ml, and the PSA level – 2.52 – 4.35 ng/ml, average maximum urination rate – 2.1 – 9.8 ml/sec. In a subjective assessment of the condition, the average IPSS score was 13.2, and QoL was 4.6 points. When treated with afala, these indicators decreased to 6.5 and 2 points, respectively. Other indicators also improved: the average volume of the prostate gland decreased slightly - it became 16.4-49.8 ml, the PSA level decreased to 2.2-3.51 ng/ml, the average residual urine amount became 4.8-6 ng/ml, and the maximum urinary flow rate increased to 3.7-13.8 ml/sec. In addition, none of the subjects reported any side effects.
The second study was conducted at clinical centers in several cities. Three groups were created among the subjects: those taking placebo (55 people), taking Afala (132 patients) and an open comparison group (54 men), the total number of subjects was 241 people. The duration of treatment in the first group was 4 weeks, in the second – 16 weeks, where patients received placebo or afala, respectively, two tablets four times a day, and in the open comparison group patients were asked to take Prostamol Uno at a dose of 320 mg/day.
The study also included patients aged 40–70 years (mean age 63.5 ± 0.49 years) with moderate symptoms of BPH with a prostate volume of more than 25 cm3, the maximum urine flow rate was 5–15 ml/sec. Patients were excluded from the study based on the following criteria: residual urine volume more than 150 ml, suspicion of prostate cancer, early surgical interventions on the prostate or bladder, serum PSA level more than 4 ng/ml.
The effectiveness of treatment with these drugs was assessed by improving the quality of life of patients during treatment, reducing the severity of symptoms of the disease, and improving urodynamic parameters.
Four weeks after the start of the study, the effectiveness indicators in the second group differed significantly from the first both in urodynamics and in the clinical manifestations of BPH. In the placebo group, the decrease in IPSS was 0.9 ± 0.4 points, Qmax did not change, and in the afala group, after four weeks of treatment, the decrease in IPSS was 1.9 ± 0.3 points, Qmax increased by 13.3 ± 2.4% .
Thus, both drugs significantly reduced the overall score by week 16; it decreased by more than 40%, and in half the IPSS decreased below 8, that is, it reached the level where pharmacotherapy is no longer carried out. The improvement in urodynamics in the Afala group was 45%, in the Prostamol group – 36%. The improvement in quality of life was also slightly more pronounced in the Afala group.
In addition, according to the results of TRUS, in the afala group by the end of treatment there was a significant decrease in the volume of residual urine (from 31.0 ± 3.2 to 12.9 ± 1.7 ml, p <0.001) and a small but significant decrease in prostate volume (from 44.6 ± 1.3 to 41.9 ± 1.3 cm3, p <0.01).
The administration of Afala at a dose of eight tablets per day for 16 weeks was also safe for patients. Just like in the first study, there were no cases of side effects or adverse events when taking this drug. The indicators of a general blood test, a general urinalysis and a biochemical blood test were within normal values both before the start of treatment and at the end of it.
In general, in patients from the afala group, the levels of free and total PSA in the blood serum remained within normal values, and in some cases they completely decreased - by 17.6 and 21.7%, respectively. There were no changes in the free PSA and total PSA ratios during treatment with afala - they remained within normal values. In the third group - in the prostamol group - the PSA content in the blood serum did not change.
Thus, as shown by the results of two controlled randomized clinical trials, afala is an effective and safe treatment for patients with BPH. When using this drug in patients with BPH, there is a decrease in the amount of residual urine and an improvement in urodynamic parameters, which in turn is associated with an improvement in bladder trophism and its function.
All of the above suggests that the drug Afala, containing ultra-low doses of antibodies to a specific prostatic antigen, is a choice for conservative treatment of BPH with moderate urinary disorders due to the elimination of irritating symptoms and good tolerability of treatment.
Topics and tags
BPH
Afala
Comments
To post comments you must log in or register
Effect of the drug
After taking a dissolving tablet, the active substances in its composition are exposed to saliva
They break down in the mouth. They are absorbed through the hypoglossal artery and are carried through the bloodstream, producing a systemic effect. In particular, getting into the tissue of the prostate gland, they accelerate metabolic processes and normalize blood circulation in the tissues. Against this background, the likelihood of vascular spasms decreases.
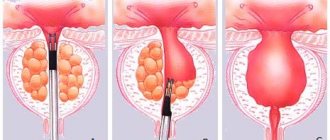
When treated with the drug at the initial stage of development of prostate adenoma, it is possible to:
- Restore damaged organ cells.
- Gradually reduce the size of the prostate gland.
- Remove blood stagnation in the pelvic area.
In addition, the active substances in the drug have the ability to liquefy the ejaculate. This increases sperm activity and eliminates the problem of infertility. In addition, afolase tablets, reviews indicate that they improve urination and relieve pain.
Indications and contraindications
The drug afalase, the instructions warn about this, is approved for use from the age of 18 years. Most often it is prescribed for the treatment of stage I and II prostate adenoma. It also helps in the following cases:
- For vegetative disorders during menopause. It helps relieve fatigue, prevent a decrease in physical activity and maintain libido.
- For disorders characterized by frequent urge and difficulty urinating, as well as pain and discomfort in the perineal area.
- When erectile dysfunction occurs due to various reasons.
Afalase is a popular remedy in the complex therapy of prostatitis in acute and chronic form. The product has an anti-inflammatory and analgesic effect, which speeds up the process of treating the disease. Afalase is also prescribed in combination with other medications before and after prostate surgery.
A contraindication for using the product is individual intolerance. This is manifested by the occurrence of negative reactions of the body. In this case, you should immediately stop taking the drug and consult a doctor to select an effective analogue. Lactose is used in the production of afalase tablets. Therefore, there are contraindications for patients with intolerance to this substance.
Afalaza tablet d/rassas x100
Afalaza tablet d/rassas x100 Trade name: AFALAZA
Pharmacological group: benign prostate hyperplasia treatment Pharmacological group for ATC: G04BE. Drugs for the treatment of erectile dysfunction Pharmacodynamics: Affinity purified antibodies to prostate-specific antigen (PSA) modify the functional activity of endogenous PSA, altered in benign prostatic hyperplasia, which is accompanied by an increase in the regulatory influence of this antigen on functional and metabolic processes in prostate tissue. Antibodies to PSA have a preventive and therapeutic effect in prostate adenoma. Antibodies to endothelial NO synthase, affinity purified, help increase the speed of blood flow in the vessels of the penis and prostate gland, have a protective effect on the endothelium (helps reduce vascular reactivity, reduce vascular spasm and improve peripheral microcirculation). The combined use of components in the complex drug "Afalase" is accompanied by a synergistic effect: antibodies to endothelial NO synthase, due to their endothelial protective effect and improved vascularization, enhance the antiproliferative and anti-inflammatory activity of antibodies to PSA. The synergistic effect is probably also due to nonspecific mechanisms of enhancing intracellular signal transduction by dilutions of antibodies to endothelial NO synthase. The complex use of the drug components helps improve the quality of life of patients with benign prostatic hyperplasia (BPH) and prostatitis, reducing dysuric disorders and erectile dysfunction, and has a vegetative stabilizing effect. The drug has been experimentally shown to have a pronounced anti-inflammatory and anti-edematous effect. The drug helps to normalize the functional state of the prostate and lower urinary tract, improve urodynamics (decrease in residual urine volume, increase maximum urine flow rate), normalize PSA levels. The drug also helps to reduce dysuric disorders, and in some cases, a moderate reduction in the volume of the prostate gland. The combined use of components improves spermatological parameters (increasing the concentration of sex hormones, the number of sperm and their motility, reducing the viscosity of seminal fluid, normalizing prostate secretions), activates regenerative and repair processes in patients who have undergone surgery for benign prostatic hyperplasia, reduces the likelihood of developing complications after surgery.
Pharmacokinetics: The sensitivity of modern physicochemical methods of analysis (gas-liquid chromatography, high-performance liquid chromatography, gas chromatography-mass spectrometry) does not allow assessing the content of ultra-low doses of antibodies in biological fluids, organs and tissues, which makes it technically impossible to study the pharmacokinetics of the drug Afalase.
Indications for use: Benign prostatic hyperplasia stages I and II. As part of complex therapy for acute and chronic prostatitis - as an anti-inflammatory and analgesic. Dysuric disorders (frequent urge to urinate, difficulty urinating, pain and discomfort in the perineal area). Erectile dysfunction (erectile dysfunction) of various origins. Autonomic disorders of menopause in men (weakness, fatigue, decreased physical activity, decreased libido, etc.). Complex therapy used before and after surgical interventions on the prostate gland.
Contraindications: Increased individual sensitivity to the components of the drug.
Dosage regimen: Inside. For one dose - 2 tablets (keep in mouth until completely dissolved - not during meals). Take the drug twice a day, in the evening and in the morning. The recommended duration of taking the drug is 16 weeks. For severe pain and dysuric disorders in the first 2-3 weeks of therapy, taking the drug up to 4 times a day is recommended. If necessary, on the recommendation of a doctor, it is possible to carry out a second course of treatment after 1-4 months.
Side effects: When using the drug according to the indicated indications and in the indicated dosages, no side effects were detected.
Overdose: In case of accidental overdose, dyspepsia may occur due to the excipients included in the drug.
Interaction: Cases of incompatibility with other drugs have not been reported to date.
Special instructions: The drug contains lactose, and therefore it is not recommended for use in patients with congenital galactosemia, glucose or galactose malabsorption syndrome, or congenital lactase deficiency. Afalase does not affect the ability to drive vehicles and other potentially dangerous mechanisms.