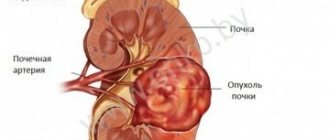
Prostate cancer (PCa) is one of the most common malignant neoplasms in men over 50 years of age. In most countries of Europe and North America, prostate cancer ranks 2nd, and in the USA it ranks 1st in the structure of cancer diseases among men. The rate of increase in the incidence of prostate cancer is one of the highest among all malignant neoplasms [1]. A significant prevalence of prostate cancer is also observed in Ukraine. Pathology ranks 4th among all cancer diseases in men and 3rd in the structure of mortality of the population of Ukraine over 60 years of age [2].
A significant increase in incidence has been noted over the past two decades. This is largely due to improved early diagnosis of the disease, primarily due to the widespread introduction into practice of determining serum prostate specific antigen (PSA) and transrectal multifocal biopsy. In Ukraine in 2002, stage I-II of the disease accounted for 39.9% of newly diagnosed cases of prostate cancer, and already in 2012 - 43.9% [2].
One of the most effective methods of treating patients with localized and locally advanced prostate cancer is radical prostatectomy (RP). According to world literature, in 29–59% of cases of localized prostate cancer, extraprostatic spread is observed during pathomorphological examination of surgical material, so this category of patients cannot always be cured with surgical treatment alone [3].
Difficulties in accurately establishing the stage of prostate cancer are primarily associated with the insufficient sensitivity of standard preoperative examination methods, which leads to underestimation of the extent of the disease in almost half of the cases [4]. On the other hand, in 15–40% of patients with localized prostate cancer, the reasons for disease progression are unknown [5]. In general, disease recurrence after RP occurs in 27–53% of cases [6–8].
Taking into account that the results of RP still cannot be considered ideal, a certain category of patients requires adjuvant hormonal and radiation therapy, the early implementation of which can significantly improve treatment results [9, 10]. Therefore, it remains relevant to identify groups of patients with a high risk of developing relapse after radical treatment for multimodal therapy, the identification criteria for which have not been developed to date.
A promising direction in predicting the course of prostate cancer is the study of serum and tissue molecular biological markers, which allows one to obtain objective information about the state of the processes of proliferation, apoptosis, and neoangiogenesis. Molecular biological markers reflect the state of stromal-epithelial relationships in the tumor, the mechanism of development of hormone resistance and androgen-independent pathways of disease progression. This allows the use of molecular biological markers when choosing a rational treatment method.
The most important marker in the diagnosis and prognosis of prostate cancer is PSA. In the case of the development of a neoplastic process in the prostate tissue, an increase in the level of PSA in the blood serum occurs due to its penetration through the basement membrane destroyed by the tumor into the extracellular space and vascular network. This leads to the fact that the level of PSA in the serum correlates with the detection rate of prostate cancer [11], and in the case of an established diagnosis of cancer, it reflects the extent of the tumor process and the response to treatment.
Unlike serum markers, which are an important tool for diagnosis, monitoring the effectiveness of treatment and early detection of disease relapses, cellular markers are determined in tumor tissue. Most of them characterize certain biological features of the tumor, the specifics of its “behavior” and regulation, for example, hormonal sensitivity or tendency to invasion and metastasis. For some molecular markers, the specific biological function has not yet been established. Their main role is that, by characterizing the biological characteristics of the tumor, they can help in predicting the course of the disease and in individualizing the approach to treatment [12].
When analyzing the world literature on the study of new immunohistochemical markers for the prognosis of prostate cancer, the markers Ki-67, p27 and ERG were selected as promising in the study of predicting the course of this disease after radical surgical treatment [15, 17, 18, 21, 23, 26].
The best known among tissue markers is Ki-67. It is used as a universal marker of proliferation in assessing the growth of malignant tumors, including prostate cancer [11]. Ki-67 expression is an independent indicator of the prognosis of relapse and survival in patients with prostate cancer [11, 13]. There is a direct correlation between the number of tumor cells expressing Ki-67 and the stage of prostate cancer, the Gleason grade of the tumor, tumor size, the presence of prostatic intraepithelial neoplasia (PIN) and the level of total PSA [11, 14-16].
The p27 marker belongs to the Kip family proteins (Kip1), is a tumor suppressor and inhibits all types of cyclin-dependent kinase, preventing the cell from entering the next phase of the division cycle. Mutations of the genes encoding p27 are quite common in cancer patients and are associated with an unfavorable prognosis of the disease. A decrease in p27 expression is an unfavorable prognostic sign and correlates with the duration of relapse-free course, survival, degree of local invasion, and damage to regional lymph nodes [15, 17, 18].
One of the promising markers from the ETS
(specific erythroblast-transforming gene), used in the prognosis of malignant neoplasms, is ERG (ETS-related gene).
The marker is responsible for the regulation of embryonic development, cell proliferation, differentiation, angiogenesis, inflammation and apoptosis [19–21]. A promising approach to determining the degree of malignancy is the study of a specific genetic mutation in prostate cancer—the product of the TMPRSS2:ERG
[22].
The detection of this marker correlates with a more aggressive form of cancer, a high degree of tumor progression and high mortality rates [23, 24]. TMPRSS2:ERG
fusion was detected in 49.2% of cases of 118 primary tumors studied and in 41.2% of hormone-independent lymph node metastases [25].
Also, TMPRSS2:ERG
can be detected in urine, the specificity of detection is more than 90%, and in 94% of cases it allows to achieve positive results in detecting prostate cancer [26].
The purpose of the study was to study the clinical significance of new molecular markers ERG, p27, Ki-67 in predicting the course of localized prostate cancer.
Material and methods
A retrospective study of the immunohistochemical markers ERG, p27 and Ki-67 was conducted at the Institute of Urology of the Academy of Medical Sciences of Ukraine and the Urological Oncology Department of the Kherson Regional Oncology Center. Of the 208 patients who underwent RP from 2002 to 2011, 44 patients with localized prostate cancer (T2N0M0) who did not receive neoadjuvant hormonal therapy were selected.
All patients underwent general clinical laboratory tests, prostate biopsy with morphological verification, PSA level determination, ultrasound, MRI or CT of the pelvic organs, and additional examinations as indicated. After surgery, a pathomorphological examination of the removed specimen was performed to determine the degree of tumor differentiation according to Gleason.
Serum PSA levels were monitored every 3 months after surgery. The presence of biochemical relapse was defined as an increase in total PSA levels to 0.2 ng/ml or higher. The study group included 16 patients with biochemically confirmed recurrent PCa who were prescribed adjuvant hormonal and/or radiation therapy. The control group included 28 patients without signs of disease relapse. The groups were comparable in age, disease stage (pTNM), PSA level, and Gleason grade of tumor differentiation (Table 1).
Table 1. Baseline data of patients by age, preoperative PSA level and Gleason grade of tumor
The observation period for patients was 42.7±4.0 (24-60) months. On average, relapse of the disease occurred after 8.3±2.4 (3-24) months. A PSA level higher than 15 ng/ml, a tumor grade of Gleason differentiation higher than 7, positive ERG expression, absence or low expression of p27, and high Ki-67 titers were considered unfavorable prognostic factors.
All patients underwent immunohistochemical examination of the surgical material to determine the level of expression of ERG, p27 and Ki-67 markers. The sections were placed on Super Frost Plus adhesive glasses (Germany). Antigenicity restoration was performed in a DAKOPT Module apparatus in TRSHighpH buffer (DAKO, Denmark), at a temperature of 97 °C for 20 min. After this, painting was carried out using a DAKO Autostainer Plus. Primary antibodies were used: to the ERG protein - monoclonal rabbit clone EP111 (Denmark), to p27Kip1 proteins - monoclonal mouse clone DCS-72.F6 (USA), to Ki-67 proteins - monoclonal mouse clone MIB-1. The DAKO FLEX+ system (Denmark) was used as a defect system. Stained sections were placed in cover medium (Italy).
The examination of the preparations was carried out using an Olympus CX31 microscope, and photography was carried out using an Olympus DP-50 digital camera. There is no universal assessment of immunostaining for ERG and p27, and the study used a qualitative assessment of response. Morphometric calculation of the index of positivity for Ki-67 was carried out on micrographs of the tumor using the Olympus DP Soft program and was determined depending on the number of tumor cells producing this marker.
Statistical analysis was performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences were considered significant at p
<0,05.
- Magazine archive /
- 2020 /
Correlation of PSA nadir level and biochemical recurrence after total HIFU ablation in patients with localized prostate cancer
DOI: https://dx.doi.org/10.18565/urology.2020.4.79-83
R.N. Fomkin, G.E. Krupinov, A.A. Churakov, T.V. Shatylko, O.A. Fomkina, V.A. Zhmakin
1) Research Institute of Fundamental and Clinical Uronephrology of the Federal State Budgetary Educational Institution of Higher Education “Saratov State Medical University named after. V. I. Razumovsky" Ministry of Health of Russia, Saratov, Russia; 2) Institute of Urology and Human Reproductive Health Federal State Autonomous Educational Institution of Higher Education “First Moscow State Medical University named after. THEM. Sechenov" of the Ministry of Health of Russia (Sechenov University), Moscow, Russia
Objective: To study the survival of patients without biochemical recurrence according to the Stuttgart and Phoenix criteria in terms of their correlation with four different PSA nadir values as predictors of clinical recurrence in patients with localized prostate cancer (PC) undergoing total HIFU ablation of the prostate. Materials and methods. The object of the study was patients with morphologically proven localized RP based on biopsy results, who underwent RP treatment using HIFU ablation using the Ablatherm Integrated Imaging® device (EDAP TMS, France). The study included 658 patients in whom HIFU ablation was used as the primary treatment for localized cancer (stages T1–T2) without previous use of other methods (hormonal, radiation therapy). A complete sample of patients was selected for analysis, divided into four groups depending on the nadir PSA level: ≤0.2 ng/ml (group 1), 0.21–0.5 (group 2), 0.51– 1 (group 3) and >1 ng/ml (group 4). Sensitivity, specificity, predictive value, and 5-year biochemical recurrence-free survival according to the Stuttgart and Phoenix definitions were assessed in PSA nadir groups. Results. The median (range) follow-up period for patients was 5.3 (3–7) years, the average period until PSA nadir was reached was 14.5 ± 2.6 weeks. PSA nadirs≤0.2, 0.21–0.5, 0.51–1.0 and >1 ng/ml were achieved by 231 (35.1%), 132 (20.0%), 105 (15. 9%) and 190 (28.8%) patients, respectively. Biochemical recurrence-free survival according to the Stuttgart definition in the four PSA nadir groups was 82%, 65%, 43% and 32%, respectively (p Conclusion: This study confirms that PSA nadir after HIFU ablation predicts biochemical recurrence-free survival and serves as a reliable marker that can be easily integrated into routine clinical practice.
Key words: PSA nadir, biochemical recurrence, HIFU, prostate cancer
The full text of the article is available in the Doctor's Library
Literature
1. Popkov VM, Fomkin RN, Blumberg BI Postoperative monitoring of prostate-specific antigen (PSA) after treatment by the high-intensive focused ultrasound (HIFU). Saratov Journal of Medical Scientific Research 2012;(4):1001–1007. Russian (Popkov V.M., Fomkin R.N., Blumberg B.I. Postoperative monitoring of prostate-specific antigen (PSA) after treatment with high-intensity focused ultrasound (HIFU). Saratov Medical Scientific Journal 2012; (4): 1001–1007) .
2. Catton C., et al. Post-Operative radiotherapy following radical prostatectomy. Euro Urol Update. 2005;3(2):107–116.
3. Fomkin RN, Popkov VM, Blyumberg BI, Bromberg BB Efficiency of high-intensity focused ultrasound ablation in the treatment of a cancer prostate of high degree oncologic risk. Herald of the Russian Academy of Military Medicine 2013; (4): 55–60. Russian (Fomkin R.N., Popkov V.M., Blumberg B.I., Bromberg B.B. Efficiency of high-intensity focused ultrasound ablation in the treatment of prostate cancer of high oncological risk. Bulletin of the Russian Military Medical Academy 2013; (4) : 55–60).
4. Uchida T., Illing RO, Cathcart PJ, Emberton M. To what extent does the prostate-specific antigen nadir predict subsequent treatment failure after transrectal high-intensity focused ultrasound therapy for presumed localized adenocarcinoma of the prostate? BJU Int 2006; 98:537–539.
5. Gelet A., Chapelon JY, Poissonnier L., et al. Local recurrence of prostate cancer after external beam radiotherapy: early experience of salvage therapy using high-intensity focused ultrasonography. Urology 2004;63:625–629.
6. Brooks JP, Albert PS, Wilder RB, et al. Long-term salvage radiotherapy outcome after radical prostatectomy and relapse predictors. J Urol 2005;174:2204–2208.
7. Pasticier G., Chapet .O, Badet .L, et al. Salvage radiotherapy after high-intensity focused ultrasound for localized prostate cancer: early clinical results. Urology 2008;72:1305–1309.
8. D'Amico AV, Moul J, Carroll PR, et al. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol 2003;21:2163–2172.
9. Glybochko PV, Fomkin RN, Blumberg BI High-intensity focused ultrasound in treatment of elderly and senile patients with prostate cancer. Clinical gerontology 2011;(9):27–33. Russian (Fomkin R.N., Glybochko P.V.,
Bloomberg B.I. Treatment of prostate cancer in elderly and senile patients with high-intensity focused ultrasound. Clinical Gerontology 2011; (9): 27–33).
10. Scher HI, Eisenberger M, D'Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 2004;22:537–556.
11. Hanlon AL, Diratzouian H., Hanks GE Posttreatment prostate-specific antigen nadir highly predictive of distant failure and death from prostate cancer. Int J Radiat Oncol Biol Phys 2002;53:297–303.
12. Murat FJ, Poissonnier L, Rabilloud M, et al. Mid-term results demonstrate salvage high-intensity focused ultrasound (HIFU) as an effective and acceptably morbid salvage treatment option for locally radiorecurrent prostate cancer. Eur Urol 2009;55:640–649.
13. Fomkin RN, Voronina ES, Popkov VM et al. Pathomorphosis of a prostate cancer at treatment by the high-intensity focused ultrasound (HIFU). Oncourology 2013; (1): 55–62. Russian (Fomkin R.N., Voronina E.S., Popkov, V.M. et al. Pathomorphosis of prostate cancer during treatment with high-intensity focused ultrasound (HIFU). Oncourology 2013; (1): 55–62.
14. Fomkin RN, Blumberg BI.. Use of the HIFU robot in a prostate cancer therapy. Uralmedical Journal 2012; (3): 48–52. Russian (Fomkin R.N., Blumberg B.I. Use of the HIFU robot in the treatment of prostate cancer. Ural Medical Journal 2012; (3): 48–52).
15. Fomkin RN, Voronina ES, Popkov VM et al. Three-year results of treatment of the localized cancer of a prostate the high-intensity focused ultrasound. Urologiia. 2014; (1): 37–44. Russian (Fomkin R.N., Voronina E.S., Popkov, V.M. et al. Three-year results of treatment of localized prostate cancer with high-intensity focused ultrasound. Urology 2014; (1): 37–44).
16. Fomkin RN, Voronina ES, Popkov VM et al. Predictive value of molecular and biological, morphological and clinical markers in an assessment of effectiveness of treatment of the localized cancer of a prostate the high-intensity focused ultrasound. Experimental and clinical urology 2013; (4): 29–33. Russian (Fomkin R.N., Voronina E.S., Popkov, V.M. et al. Prognostic value of molecular biological, morphological and clinical markers in assessing the effectiveness of treatment of localized prostate cancer with high-intensity focused ultrasound. Experimental and Clinical Urology 2013; 4:29–33).
17. Maslykova GN, Fomkin RN, Voronina ES Morphological methods of research in diagnostics of a cancer of a prostate. Basicresearches 2012; (12): 426–430. Russian (Maslyakova G.N., Voronina E.S., Fomkin R.N. Morphological research methods in the diagnosis of prostate cancer. Fundamental Research 2012; 12 (2): 426–430).
18. Glybochko PV, Fomkin RN, Blumberg BI et al. The first experience of using high-intensity focused ultrasound ablation (HIFU) treatment of prostate cancer. Saratov Journal of Medical Scientific Research 2009; 5(4): 599–603. Russian (Glybochko P.V., Fomkin R.N., Blumberg B.I. et al. The first experience of using high-intensity focused ultrasound ablation (HIFU) in the treatment of prostate cancer. Saratov Medical Scientific Journal 2009; 5(4): 599 –603).
19. Popkov VM, Fomkin RN, Blumberg BI High-intensity focused ultrasound (HIFU) – an alternative method of treatment of cancer. Saratov Journal of Medical Scientific Research 2012; (4):989-995. Russian (Popkov V.M., Fomkin R.N., Blumberg B.I. High-intensity focused ultrasound (HIFU) is an alternative method for the treatment of urological diseases. Saratov Medical Scientific Journal 2012; (4): 989–995).
20. Blana A., Murat F. J., Walter B., et al. First analysis of the long-term results with transrectal HIFU in patients with localized prostate cancer. Euro Urol 2008; 53:1194–1203.
21. Popkov VM, Fomkin RN, Blumberg BI Possibilities of forecasting of relapse of the cancer prostate after HIFU ablation by means of mathematical modeling. Saratov Journal of Medical Scientific Research 2013; 9(2):314–320. Russian (Popkov V.M., Fomkin R.N., Blumberg B.I. Possibilities of predicting recurrence of prostate cancer after HIFU ablation using mathematical modeling. Saratov Journal of Medical Sciences 2013; 9 (2): 314–320).
22. Ganzer R., Rogenhofer S., Walter B. et al. PSA nadir is a significant predictor of treatment failure after high-intensity focused ultrasound (HIFU) treatment of localized prostate cancer. Euro Urol 2008; 53:547–553.
23. Pasquier D., Ballereau C. Adjuvant and salvage radiotherapy after prostatectomy for prostate cancer: a literature review. Int J Radiat Oncol Biol Phys 2008; 72:972–979.
24. Poissonnier L., Chapelon JY, Rouvie' re O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol 2007;51:381–387.
25. Fomkin RN, Popkov VM, Shatylko TV Comparing the definitions of biochemical recurrence after treatment of prostate cancer of high-intensity focused ultrasound. Medical bulletin of Bashkortostan 2015; (3): 199-202. Russian (Fomkin R.N., Popkov V.M., Shatylko T.V. Comparison of definitions of biochemical relapse after treatment of prostate cancer with high-intensity focused ultrasound. Medical Bulletin of Bashkortostan 2015; (3): 199–202).
26. Vallancien G., Prapotnich D., Cathelineau X. et al. Transrectal focused ultrasound combined with transurethral resection of the prostate for the treatment of localized prostate cancer: feasibility study. J Urol 2004; 171(6 Pt 1): 2265–2267.
About the authors / For correspondence
AUTHORIZATION: R. N. Fomkin – Candidate of Medical Sciences, Associate Professor, Associate Professor of the Department of Urology, Senior Researcher, Research Institute of Fundamental and Clinical Uronephrology of the Federal State Budgetary Educational Institution of Higher Education “Saratov State Medical University named after. V. I. Razumovsky" Ministry of Health of Russia, Saratov, Russia; e-mail: [email protected] ;
Similar articles
- Experimental rationale for the use of ND:YAG laser coagulation of bladder tissue
- Three-year results of treatment of localized prostate cancer with high-intensity focused ultrasound
- Erectile dysfunction after nerve-sparing radical prostatectomy
- Diagnosis and treatment of local recurrence of prostate cancer using histoscanning and high-intensity focused ultrasound in patients after radical prostatectomy
- A new method for non-invasive mechanical destruction of prostate tumors using pulsed focused ultrasound
Results and discussion
During pathomorphological examination, the degree of differentiation according to Gleason ranged from 6 to 9, including 6 in 29 (65.9%), 7 in 9 (20.5%), 8 in 4 (9.1%) and 9 - in 2 (4.5%) patients. When analyzing the data obtained, a clear dependence of PCa progression on the degree of tumor differentiation according to Gleason was noted - 9 (56.2%) of 16 patients from the study group and only 6 (21.4%) of 28 from the control group had Gleason ≥7 (Fig. 1).
Rice. 1. Dependence of the occurrence of biochemical relapse on the Gleason grade.
It was determined that in patients with biochemical relapse, a preoperative PSA level above 15 ng/ml occurred more frequently than in the group without relapse - 8 (50.0%) patients versus 11 (39.3%) (Fig. 2).
Rice. 2. Dependence of the occurrence of biochemical relapse on the level of preoperative PSA.
Positive expression of the ERG marker was found in 20 patients and occurred with the same frequency in both groups - 7 (48.8%) patients in the study group and 13 (46.4%) in the control group (Fig. 3).
Rice. 3. Dependence of the occurrence of biochemical relapse on ERG expression.
Low p27 expression is characterized by a worse prognosis for prostate cancer. The obtained data noted the absence of p27 expression in 4 (25.0%) patients with biochemical relapse and in 6 (21.4%) patients without relapse. Similar results were obtained when comparing patients with a weak and moderate positive reaction [5 (31.3%) patients in the study group versus 8 (28.6%) in the control group]. Only with high positive expression of p27+++, which is characterized by a potentially favorable prognosis, was the expected result obtained. High expression of p27+++ was 3 times less common in patients of the study group than in patients of the control group - 2 (12.5%) patients versus 6 (21.4%) (Fig. 4).
Rice. 4. Dependence of the occurrence of biochemical relapse on the level of p27 expression.
Thus, the study did not find a significant effect of positive expression of the ERG marker and negative or low expression of p27 on the prognosis of prostate cancer. Considering the undoubted importance of the above markers in carcinogenesis, further study of their significance in prognosis of prostate cancer is necessary. There is a tendency towards a higher frequency of determining a strongly positive p27+++ reaction in patients without relapse of the disease, which may indicate a favorable prognosis for the course of the disease with high expression of this marker.
High expression of the Ki-67 marker is characterized by a poor prognosis for prostate cancer. A Ki-67 level above 20% is considered especially unfavorable for the course of malignant neoplasms [27, 28]. In patients with biochemical relapse, the Ki-67 expression level ranged from 2.40-27.12, with an average of 11.46±1.57; in the group without relapse, this figure was 4.17-17.83, with an average of 8. 36±0.73, i.e. in patients with biochemical relapse, high Ki-67 numbers were observed more often. Expression of Ki-67 above 20% was detected in only one case; this patient had a relapse of the disease (Table 2).
Table 2. Dependence of the presence of biochemical relapse on prognosis markers for prostate cancer
Cancer that has clearly spread
If the cancer has spread beyond the prostate, it will likely spread first to nearby lymph nodes and then to the bones. It is much less likely that cancer will spread to the liver or other organs.
When prostate cancer has spread to other parts of the body, hormone therapy is probably the most effective treatment. But it is unlikely to cure cancer, and at some point it may stop working. Usually the first treatment is LHRH. Another option may be to receive chemotherapy along with hormone therapy. Other treatments aimed at treating bone metastasis may also be used.