Indications for BCG therapy
If there is a high probability of relapse of the disease and transition to an invasive form, they resort to administering the BCG vaccine into the bladder cavity. These may be the following cases:
- Papillary cancer (T1) of the second degree and higher, with a tumor size of more than 3 cm, or in the presence of several formations.
- Papillary cancer (Ta and T1) of the third degree.
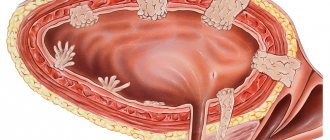
- Carcinoma in situ – it has a number of features. Firstly, it is flat, which makes it difficult to remove it surgically. Secondly, it often grows into the bladder mucosa.
Specialists from our partner clinics will definitely explain to the patient why BCG therapy in this case is justified and appropriate and is the most appropriate treatment. For those who have undergone surgery, a break of more than two weeks is taken to allow for recovery.
Bladder cancer (BC) remains a pressing problem in urological oncology. This is primarily due to high morbidity and mortality rates from this pathology. Every year, up to 200 thousand new cases of bladder cancer are registered worldwide. In the structure of cancer incidence, bladder cancer ranks 4th in men and 10th in women. In Russia, morbidity and mortality rates from this pathology remain disappointing. Thus, in 2009, the “crude” incidence rate was 9.34 per 100 thousand population, the increase over the last 10 years was 14.85%, 13,260 new cases were registered, 6,933 patients died [1, 2].
Non-muscle-invasive (MNI) bladder cancer is detected in 70% of patients, and in a third of patients from this group, a morphological study verifies the papillary form of stage Ta without invasion into the subepithelial base, and in 30% - T1 or carcinomainsitu (CIS) [3]. Frequent relapses of the disease, especially during the first year after transurethral resection (TUR), as well as continuous progression of the tumor process are the main problems in the treatment of patients with MNI BC. Taking this fact into account, preventive measures aimed at reducing the number of relapses remain relevant [4].
The occurrence of relapse or progression of the disease depends on a number of prognostic factors. Thus, relapse is observed in 30% of cases in the presence of a single tumor, and its frequency reaches 90% in multifocal lesions. Depending on the size of the tumor, progression is observed in 10% of cases with tumors < 3 cm and in 35% with tumor sizes ≥ 3 cm [3, 5]. As a result of the systematization of the main prognostic factors, the European Organization for Research and Treatment of Cancer (EORTC) developed tables and nomograms that allow dividing patients into low, intermediate and high risk groups [6].
As a result, the so-called risk-associated therapy. According to this concept, all patients, regardless of the risk group, after TUR (within the next 6 hours) are advised to perform an immediate single intravesical instillation of a cytostatic agent. In a meta-analysis of 7 randomized trials (1476 patients with a median follow-up of 3.4 years), a single immediate administration of chemotherapy after TUR reduced the relapse rate by 12% (from 48.4 to 36.7%) and the chance of relapse by up to 39%. The effect was confirmed for both single and multiple tumors [7]. Patients with a low risk of relapse or progression (EORTC score 0) after surgery and a single instillation are advised to undergo careful follow-up [8]. However, since the likelihood of relapse and progression remains significant, for other patients (2 or more EORTC points) this amount of treatment is insufficient. For patients with intermediate risk, several courses (4–8) of intravesical chemotherapy (IVCT) or immunotherapy are indicated [9, 10]. Patients with a high risk of recurrence or progression are advised to undergo a repeat TUR of the bladder in the period from 2 to 6 weeks after the first TUR (second look operation) and immunotherapy with the BCG vaccine for 1–3 years [11]. At the same time, based on an analysis of a number of large randomized clinical trials that compared various HFCT regimens, RJ Sylvester et al. believe that the duration and intensity of adjuvant HICT remain uncertain due to conflicting data [12].
Intravesical chemotherapy
In the early 1990s. MF Sarosdy [13] characterized the optimal chemotherapy drug for HPCT as a drug that effectively prevents the risk of relapse and progression of the disease; having minimal toxicity, reasonable cost and a constant dose per administration. Of the number of currently used cytostatics, such as epirubicin, doxorubicin and mitomycin, the latter is the most studied, effective and at the same time convenient to use. It is an alkylating chemotherapeutic drug isolated from the fungus Streptomyces caespitosus and is a natural cytotoxic antibiotic with a molecular weight of 334.4 kDa. Mitomycin inhibits the synthesis and damages DNA in cells, and also promotes peroxidation of the lipid membrane. It is an effective cytostatic agent that can be used both as a single instillation after TUR and for long-term HICT.
The results of an analysis of relapse-free survival after TUR of the bladder and VPCT in a comparative study that included more than 2500 patients with MNI bladder cancer demonstrated a reduction in the risk of disease recurrence by 15% during the first two years of follow-up compared with TUR alone [14].
The duration of intravesical instillation of mitomycin is an important aspect when performing VPCT. Long-term exposure of the chemotherapy drug to the bladder cavity allows it to penetrate deeper into the mucous membrane, which provides a good cytostatic effect, but the likelihood of toxic reactions increases. According to AA Giesbers et al., 60-minute intravesical instillation is optimal. The incidence of side effects is minimal [15].
A number of authors have shown that HFCT with mitomycin is an effective and safe method for preventing relapse in patients with MNI BC (Table 1).
. Relapse rate during intravesical mitomycin therapy.
Recently, in urological oncology practice, quite a lot of attention has been paid to methods for increasing the effectiveness of intravesical instillations of cytostatics. The European Association of Urology (EAU – European Association of Urology) recommends preventing dilution of the chemotherapy drug and alkalinizing the urine when performing VPCT [3]. The use of such physical factors as electrodiffusion, microwave hyperthermia in combination with mitomycin (Mitomycin C) significantly increases the effectiveness of HPCT [16, 17] (Table 2).
. Comparison of the effectiveness of various regimens of HPCT with mitomycin.
According to some authors, the combined use of mitomycin and photodynamic therapy (PDT) can increase the bioavailability of tumor cells to various chemotherapeutic agents [19]. For example, in the work of AJ French et al. The effectiveness of PDT and mitomycin was analyzed on a cell line model of a mitomycin-resistant bladder tumor. The authors conclude that the combined use of PDT and mitomycin can help reduce the risk of relapse in patients with superficial bladder cancer. However, further research in this area is necessary [20].
In turn, RJ Skyrme et al. presented phase I clinical trials to evaluate the effectiveness of combination therapy using PDT and mitomycin in patients with recurrent MNI BC. The authors did not note increased photosensitivity or urinary incontinence after the procedure in any patient. In addition, relapses of the disease over two years of follow-up were observed in only 40% of patients. The researchers concluded that combination therapy with mitomycin and PDT is an effective and safe treatment for both recurrent MNI bladder cancer and CIS [21].
In the work of E. Solsona et al. the combined use of mitomycin and BCG significantly increases relapse-free survival compared to monotherapy with the BCG vaccine (p = 0.03) and can be used in a group of high-risk patients. However, the toxic manifestations of such a scheme are pronounced, which, according to the authors, requires further study [18].
Recently, a new domestic mitomycin preparation, Vero-Mitomycin (Veropharm, Russia), has appeared on the market. The drug is available in convenient packaging in a 20 mg bottle. Before intravesical instillation, 2 vials of VeroMitomycin are diluted in 40 ml of physiological solution. The effectiveness of the drug Vero-Mitomycin and the incidence of side effects are identical to those when using mitomycin.
Adjuvant immunotherapy with BCG vaccine
The use of BCG therapy after TUR in patients at high risk of relapse and progression is considered the only effective method of prevention [22, 23]. At the same time, in 20% of patients, the BCG course is canceled due to local and systemic toxicity, and approximately 30% of patients experience relapses of the disease [28].
A number of retrospective studies have demonstrated long-term disease-free survival, as well as a significant reduction in the incidence of progression after BCG therapy [24–25]. Six clinical trials comparing TUR alone and TUR followed by intravesical immunotherapy with BCG vaccine found a significant benefit to BCG therapy [26–27] (Table 3).
. Efficacy of intravesical BCG therapy in controlled studies.
Randomized clinical trials comparing BCG immunotherapy with IPCT in a group of high-risk patients also found a statistically significant reduction in the incidence of tumor recurrence with BCG compared with doxorubicin and mitomycin [28–31] (Table 4).
. Relapses in comparative controlled studies.
In a multicenter prospective randomized study of patients with primary T1 bladder cancer of stages II–III, M. Duchek et al. compared BCG therapy with a combination of epirubicin and interferon alfa-2 as adjuvant therapy. The study included 250 patients who were randomized into two groups. In each group, patients received intravesical instillations for 6 weeks followed by maintenance therapy for two years, after which the time to relapse and progression was compared. Additionally, toxic reactions of the therapy were recorded. In the group of patients with combination treatment, relapse was detected in 38% of cases, while in the group of monotherapy with the BCG vaccine - only in 17% (p = 0.012). However, no difference in the progression rate was noted. The analyzed subgroup showed the superiority of BCG therapy against in situ cancer [32].
In turn, a randomized phase III trial that included 957 patients (EORTC 30911) compared epirubicin, BCG, and BCG with isoniazid in the treatment of patients with intermediate- and high-risk MNI bladder cancer. The authors concluded that intravesical BCG therapy with or without isoniazid was superior to intravesical epirubicin therapy not only in time to first relapse, but also in time to distant metastases, overall survival, and tumor-specific survival. The benefits of BCG therapy are not limited to high-risk patients; Average risk patients also benefit from BCG [33].
The use of the BCG vaccine for 1.5–2.0 years in high-risk patients with frequent relapses is justified and recommended for clinical use by EAU. For example, R. Jarvinen et al. analyzed the long-term results of the effectiveness of continuous administration of BCG compared with continuous administration of mitomycin as intravesical therapy for frequent relapses of TaT1 bladder tumors without carcinoma in situ. The study involved 89 patients with high-risk MNI BC, included in a prospective study from 1984 to 1987 and randomized to receive BCG or mitomycin. Both regimens consisted of five weekly and subsequent monthly instillations over two years. The total duration of follow-up was 8.5 years, while the average duration of follow-up for patients still alive was 19.4 years. The main objective was to assess the time to first relapse and overall mortality, the secondary objective was to assess the time to progression and tumor-specific mortality. A relapse was diagnosed in 36 (80.0%) of 45 patients in the mitomycin group, while in the BCG therapy group a relapse was detected in only 26 (59.1%) of 44 patients. In a general analysis of data from both groups, a trend toward improved disease-free survival (p = 0.1) and a decrease in tumor-specific mortality rates (p = 0.2) in the BCG therapy group was noted. There were no differences in overall mortality rates. The study population was too small to provide conclusive evidence of progression or survival. The authors concluded that intensive intravesical BCG therapy results in a long-lasting and significant reduction in recurrence rates for frequently recurrent bladder tumors [34].
Recently, the Uro-BCG-medac vaccine appeared on the domestic market, which is produced in the form of a system for intravesical administration with a closed circuit. When using this system, there is absolutely no contact of medical personnel with the active substance, which eliminates the risk of infection with Mycobacterium tuberculosis.
In addition, it should be noted that Uro-BCG-medac is recommended by the EAU as the drug of choice for patients with MNI BC after standard surgical treatment in the presence of a moderate and high risk of relapse or progression of the disease.
Thus, VPCT and BCG therapy are an effective method for preventing relapse and progression of the disease in groups of patients with intermediate and high-risk MNI BC. A promising direction for the adjuvant use of HPCT and BCG therapy is the combination of these methods with various physical factors (PDT, electrodiffusion, microwave hyperthermia). However, the results obtained cannot be of a recommendatory nature, since the effectiveness of these methods must be confirmed by the results of large randomized studies.
Information about the authors: Golovashchenko Maxim Petrovich – postgraduate student, Department of Oncourology, Moscow Research Institute named after. P.A. Herzen. Tel.; Rusakov Igor Georgievich – Doctor of Medical Sciences, Professor, Head of the Department of Oncourology, Moscow Research Institute named after. P.A. Herzen. Email
Description of the method
Treatment with the vaccine takes six weeks. After this, it is recommended to take a break for a month and a half and begin repeating the course again. Now once a week, one to three weeks.
Maintenance therapy is also possible if the result is good. It can be carried out for three years. The drug administration schedule is once a week. For 1-3 weeks with a break of six months.
In each specific case, the doctor will give detailed explanations and warn about all possible complications and adverse reactions.
How is the treatment carried out?
Treatment is possible on an outpatient basis. One session takes about 3 hours. After this, the patient can return to his home.
Before the session, the patient is advised to limit fluid intake so that the concentration of the drug injected into the bladder does not decrease. Your doctor will definitely tell you in more detail about the preparation and correction of the therapy taken before BCG therapy.
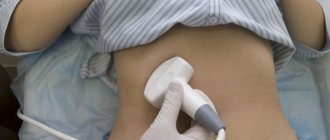
During the appointment, the patient takes a comfortable position. The nurse carefully inserts a catheter through which the drug is administered. The first quarter of an hour will need to be spent lying on your stomach, and after that you can get up and even move around. The therapeutic effect of the drug depends on the amount of time it remains in the bladder cavity. This is usually two hours. If necessary and other indications, the exposure time can be reduced, strictly in consultation with the attending physician.
You should not urinate during the procedure. This may cause some difficulties. Sometimes the catheter is left in the bladder, blocking the exit. This allows the drug not to leak out during the procedure.
At the end of the session, the patient is allowed to urinate into the toilet or the catheter opening is opened, allowing the drug to flow out.
The patient is advised to take precautions at home and in public places. Because BCG is a live vaccine, other people should not be exposed to the TB pathogen (even as it is contained in the vaccine). To do this, for the next six hours you need to make sure that urine does not splash on the toilet or hands. After urinating, the toilet should be treated with an undiluted solution to destroy vaccine residues and left for 15 minutes. The water flushes with the toilet lid down. The patient will be told about all this before and after the procedure.
How does the BCG vaccine work for bladder cancer?
BCG is one of the strongest nonspecific, or general, immunostimulants known to science. Bladder cancer is often compared to a lawn overgrown with weeds. You can pull out these weeds one at a time, like dandelions, but they will still grow back. Or you can treat the weeds with chemicals. This principle is implemented in intravesical chemotherapy. Ikhilov doctors treat the lawn with poison in the hope that the weed will die.
BCG is an organic solution to the problem. In this case, Israeli oncologists use what nature itself has provided. BCG causes a natural immune response to cancer cells, prompting the body to independently recognize and destroy the threat.
It must be taken into account, however, that BCG acts differently than the standard means of immunotherapy - vaccines. After receiving the flu vaccine, the body produces antibodies so that it does not get sick with this particular disease this year. The mechanism of action of BCG is nonspecific. This means that BCG activates the entire immune system, and immune cells rush to the bladder to fight tuberculosis. Humanity has been fighting tuberculosis throughout its history, and for many centuries the immune system has simply had no choice but to develop a response to the causative agents of the disease.
Apply to Ikhilov
Side effects
The first thing the drug affects is the bladder. Therefore, most complications relate to local reactions. Below we list the main possible complications.
- Various unpleasant or painful sensations in the bladder area can persist for up to two days. To reduce them, it is recommended to increase fluid intake and, if necessary, use an anesthetic drug.
- Less common concerns include fever , cough and joint pain . The first symptoms of such ailment should be reported to your doctor immediately. This may be a sign of infection and require immediate treatment. In case of such complications, anti-TB drugs may be required.
- Allergic reactions in the form of skin rash and arthralgia are possible
- To avoid serious complications, therapy is not given to people over 80 years of age. And it is carried out with caution for people over 70 years of age.