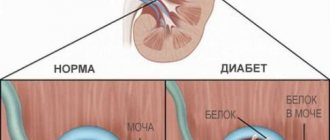
Causes and symptoms of nephropathy
Nephropathy can be caused by a number of diseases, primarily diabetes mellitus, endocrine disorders, toxic poisoning, cancer, etc.
Having arisen against the background of any disease or as a result of one or another negative impact on human health, nephropathy can develop quite slowly, at first not showing any special symptoms.
Then weakness, shortness of breath and fatigue, headaches, and pain in the lumbar region appear. A person begins to feel thirsty more often.
With the further development of nephropathy, swelling begins to appear and problems with blood pressure arise. There is a threat of chronic renal failure.
In addition, against the background of nephropathy, the risk of inflammatory kidney diseases – pyelonephritis – increases.
1
Consultation with a nephrologist in MedicCity
2 Consultation with a nephrologist in MedicCity
3 Consultation with a nephrologist in MedicCity
Purine metabolism disorders and gouty nephropathy
The concept of “gouty nephropathy” includes various forms of kidney damage caused by disorders of purine metabolism and other metabolic and vascular changes characteristic of gout. Gout affects 1–2% of the population, predominantly men [1]. If early asymptomatic disorders of purine metabolism are potentially reversible with timely diagnosis and correction, then at the stage of tophi gout with damage to blood vessels and target organs (heart, brain, kidneys), the prognosis of the disease is unfavorable. Kidney damage develops in 30–50% of patients with gout. With a persistent increase in blood uric acid levels > 8 mg/dL, the risk of subsequent development of chronic renal failure (CRF) increases by 3–10 times. Every 4th patient with gout develops chronic renal failure [2].
Both acquired and hereditary factors play a role in the development of gout. The role of malnutrition in combination with physical inactivity is especially important. Over the past 20 years, Europe and the USA have seen a multiple increase in the incidence of gout in parallel with the epidemic of morbid obesity, nephrolithiasis and non-insulin-dependent diabetes mellitus [1, 3]. Gout develops especially often in countries with high per capita consumption of meat products.
The metabolic syndrome with insulin resistance characteristic of gout, as well as hyperphosphatemia, contribute to the formation of severe atherosclerosis of the renal and coronary arteries with the development of coronary heart disease, renovascular hypertension, and the addition of calcium urate nephrolithiasis.
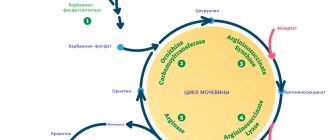
The leading pathogenetic mechanisms of gouty nephropathy are associated with an increase in the synthesis of uric acid in the body, as well as with the development of an imbalance between the processes of tubular secretion and reabsorption of urates. Overproduction of uric acid is caused by deficiency of hypoxanthine-guanine phosphoribosyltransferase (GGPT). GGPT is controlled by genes located on the X chromosome. This explains the fact that gout affects almost exclusively males. Complete deficiency of GGPT leads to Lesch–Nychen syndrome, characterized by early and especially severe gout. Other variants of juvenile hereditary gout include forms caused by a mutation of the Tamm-Horsfall tubular protein, nuclear liver factor - RCAD (renal cyst and diabetes) syndrome (a combination of gout with cystic renal dysplasia and non-insulin-dependent diabetes mellitus) [4]. Hyperuricemia is also caused by increased intracellular destruction of adenosine triphosphate (ATP): a defect characteristic of glycogenosis (types I, III, V), congenital fructose intolerance, and chronic alcoholism [1]. At the same time, the majority of patients with primary gout exhibit disturbances in the tubular function of the kidneys: decreased secretion, increased various phases of reabsorption. An important role in pathogenesis is played by the defect of tubular acidogenesis, which contributes to the crystallization of urates in the urine [2]. The defect is manifested by the formation of urine with a persistently acidic reaction (pH < 5) in gout.
The kidney-damaging effect of hyperuricosuria leads to urate nephrolithiasis with secondary pyelonephritis, urate damage to the interstitial tissue of the kidneys with the development of chronic tubulointerstitial nephritis, as well as renal acute renal failure (ARF) due to intratubular obstruction by uric acid crystals (acute uric acid nephropathy).
Hyperuricemia, due to activation of the renal reninangiotensin system and cyclooxygenase-2, increases the production of renin, thromboxane and vascular smooth muscle cell proliferation factor, and also induces atherogenic modification of very low-density lipoproteins (VLDL).
As a result, afferent arteriolopathy develops with renal hypertension and subsequent glomerulosclerosis and nephroangiosclerosis [5].
Urate nephrolithiasis. It is characterized, as a rule, by bilateral lesions, frequent relapses of stone formation, and sometimes coral nephrolithiasis. Urate stones are X-ray negative and are better visualized on echography. Outside of an attack, changes in urine tests may be absent. Renal colic is accompanied by hematuria and urate crystalluria. With prolonged renal colic, nephrolithiasis can be complicated by an attack of secondary pyelonephritis, postrenal acute renal failure. With a long course it leads to hydronephrotic transformation of the kidney, pyonephrosis.
Chronic tubulointerstitial nephritis. It manifests itself as persistent urinary syndrome, often combined with arterial hypertension. In this case, proteinuria, not exceeding 2 g/l in more than half of the patients, is combined with microhematuria. No stones are usually found, but episodes of gross hematuria with transient oliguria and azotemia, provoked by dehydration, are observed. In 1/3 of patients, bilateral medullary cysts (0.5–3 cm in diameter) are found. Typically, the early development of hyposthenuria and nocturia, as well as hypertension with glomerulosclerosis. Arterial hypertension is usually controlled. The appearance of difficult-to-control hypertension indicates the progression of glomerulosclerosis and nephroangiosclerosis or the formation of atherosclerotic stenosis of the renal arteries.
Acute uric acid nephropathy. It manifests suddenly with oliguria, dull pain in the lower back with dysuria and gross hematuria, often combined with an attack of gouty arthritis, hypertensive crisis, and an attack of renal colic. Oliguria is accompanied by the release of red-brown urine (urate crystalluria). At the same time, the concentrating ability of the kidneys is relatively preserved, and urinary sodium excretion is not increased.
In the future, oliguria quickly turns into anuria. With the aggravation of intratubular obstruction by the formation of numerous urate stones in the urinary tract and bladder, azotemia increases at a particularly high rate, which makes it possible to classify this option as an urgent form of sudden onset gouty nephropathy.
Diagnosis and differential diagnosis
Clinically, the diagnosis of gout is most likely in the development of acute arthritis against the background of manifestations of metabolic syndrome - alimentary obesity of the abdominal type in combination with volume-sodium-dependent hypertension, hyperlipidemia, hyperinsulinemia, microalbuminuria. Laboratory diagnosis of gout is based on identifying disorders of uric acid metabolism: detection of hyperuricemia (> 7 mg/dl), hyperuricosuria (> 1100 mg/day), persistently acidic urine pH, proteinuria (microalbuminuria), hematuria, crystalluria. Instrumental diagnostics include ultrasound examination (identification of X-ray negative urate stones), as well as (in difficult cases) biopsy of the affected joint, tophi. In this case, the detection of intracellular crystals of uric acid in the synovial fluid and in the contents of tophi (by polarization microscopy) is informative. Doppler ultrasound is performed for difficult-to-control hypertension in patients with gout in order to exclude atherosclerotic stenosis of the renal arteries.
The second stage of diagnosis is the distinction between gout and secondary hyperuricemia. Among the diseases often accompanied by disorders of purine metabolism are: chronic lead intoxication (lead nephropathy), chronic alcohol abuse, analgesic nephropathy, common psoriasis, sarcoidosis, berylliosis, hypothyroidism, myeloproliferative diseases, polycystic disease, cystinosis. Hyperuricemia in alcoholism, as a rule, is asymptomatic and is characterized by excess dependence [6]. It should be emphasized the unfavorable prognostic significance of hyperuricemia in pregnancy nephropathy [7], immunoglobulin A (IgA) nephropathy [8] and alcoholism [6]. Tumor lysis syndrome is a great danger: acute uric acid nephropathy, complicating chemotherapy for cancer. Chronic tubulointerstitial nephritis is characterized by hypertension, early anemia, and osteoporosis. The outcome in chronic renal failure is not uncommon. Diagnosis is based on the detection of increased concentrations of lead in the blood and urine after a test with complexones (EDTA - from the English ethylenediaminetetraacetic acid) [9]. Drug-induced secondary hyperuricemia also needs to be differentiated from primary gout. Drugs that cause hyperuricemia include: thiazide and (to a lesser extent) loop diuretics, salicylates, nonsteroidal anti-inflammatory drugs, nicotinic acid, ethambutol, cyclosporine, antitumor cytostatics and antibiotics, ribavirin. Particularly important is the diagnosis of chronic renal failure (gouty “mask” of uremia), which sharply impairs the renal elimination of uric acid [2].
Course and prognosis of gouty nephropathy
Gouty nephropathy usually occurs at one of the stages of the long-term course of chronic “tophus” gout with attacks of gouty arthritis. At the same time, in 30–40% of cases, nephropathy is the first manifestation - a renal “mask” - of gout or develops against the background of an articular syndrome atypical for gout (damage to large joints, polyarthritis, arthralgia).
Advanced gout with a risk of target organ damage is indicated by hypertension with circadian rhythm disturbances, the formation of metabolic syndrome, microalbuminuria, a significant increase in lipids (low-density lipoprotein cholesterol > 130 mg%), C-reactive protein. Among the early signs of target organ damage in gout: persistent proteinuria, a moderate decrease in glomerular filtration (up to 60–80 ml/min), left ventricular hypertrophy, and the addition of diabetes mellitus. Gouty nephropathy typically has a latent or recurrent course with bilateral renal colic (urate nephrolithiasis), repeated episodes of reversible renal acute renal failure (acute uric acid nephropathy). On average, 12 years pass from the clinical manifestation of gouty nephropathy to the appearance of chronic renal failure.
Risk factors for the development of chronic renal failure in gout include persistent arterial hypertension, proteinuria > 1 g/l, the addition of chronic pyelonephritis, diabetes mellitus, old age of the patient with gout, juvenile forms of gout, chronic alcoholism.
Treatment of gouty nephropathy
Treatment of acute uric acid nephropathy is carried out in accordance with the principles of treatment of acute renal failure caused by acute intratubular obstruction. In the absence of anuria, signs of ureteral obstruction with urates (postrenal acute renal failure) or bilateral atherosclerotic stenosis of the renal arteries (ischemic kidney disease), conservative treatment is used. Continuous intensive infusion therapy is used (400–600 ml/h) using isotonic sodium chloride solution, 4% sodium bicarbonate solution and 5% glucose, 10% mannitol solution (3–5 ml/kg/h), furosemide (up to 1. 5–2 g/day, in fractional doses). In this case, diuresis should be maintained at a level of 100–200 ml/h, and the urine pH should reach a value of 6.5, which ensures the dissolution of urates and the excretion of uric acid. At the same time, allopurinol is prescribed at a dose of 8 mg/kg/day or urate oxidase (0.2 mg/kg/day, intravenously). If there is no effect from this therapy within 60 hours, the patient is transferred to acute hemodialysis. In the event that acute uric acid nephropathy has developed as a complication of tumor chemotherapy (hemoblastosis) as part of secondary hyperuricemia - with tumor lysis syndrome, emergency hemodialysis (hemodiafiltration) together with allopurinol is immediately indicated due to the low effectiveness of conservative infusion therapy.
Treatment of chronic forms of gouty nephropathy should be comprehensive and include solving the following problems:
- correction of purine metabolism disorders;
- correction of metabolic acidosis and urine pH;
- normalization of the value and daily (circadian) rhythm of blood pressure (BP);
- correction of hyperlipidemia and hyperphosphatemia;
- treatment of complications (primarily chronic pyelonephritis).
The diet should be low-purine, low-calorie and combined with plenty of alkaline drinking (2-3 l/day). The daily quota of proteins should not exceed 1 g/kg, fats - 1 g/kg. Long-term adherence to such a diet reduces the level of uric acid in the blood by 10% (uricosuria - by 200-400 mg/day), helps normalize body weight, blood lipids and phosphates, as well as reduce metabolic acidosis. It is advisable to enrich the diet with potassium citrate or potassium bicarbonate, as well as fish oil. Eicosapentaenoic acid, the active principle of fish oil, has a nephroprotective and cardioprotective effect in gout due to its high content of polyunsaturated fatty acids. Its long-term use reduces the volume of adipose tissue, proteinuria, insulin resistance, dyslipidemia, and hypertension. For gouty nephropathy in the stage of chronic renal failure, a low-protein diet (0.6–0.8 g/kg) should be used.
Let us list the drugs that affect purine metabolism.
- Relieving gouty arthritis: colchicine; non-steroidal anti-inflammatory drugs; glucocorticosteroids.
- Xanthine oxidase inhibitors: allopurinol (milurite); urate oxidase (rasburicase).
- Uricosuric drugs: benzbromarone, sulfinpyrazone, probenecid; angiotensin II (A II) receptor blockers; statins.
- Citrate mixtures: uralite; magurlit; lemaren.
Drugs that control hypertension in gout include:
- angiotensin-converting enzyme (ACE) inhibitors;
- A II receptor blockers;
- calcium antagonists;
- selective β-blockers;
- loop diuretics;
- statins;
- fibrates.
Allopurinol (milurite) reduces the production and level of uric acid in the blood by inhibiting the enzyme xanthine oxidase. Promotes the dissolution of urates. The hypouricemic effect of allopurinol correlates with its nephroprotective effect associated with a decrease in proteinuria, renin production, free radicals, as well as a slowdown in glomerulosclerosis and nephroangiosclerosis. Indications for the use of allopurinol: asymptomatic hyperuricemia in combination with hyperuricosuria > 1100 mg/day, gouty chronic tubulointerstitial nephritis, urate nephrolithiasis, prevention of acute uric acid nephropathy in cancer patients and its treatment.
The daily dose of allopurinol (from 200 to 600 mg/day) depends on the severity of hyperuricemia. In view of the possibility of exacerbation of gouty arthritis, it is advisable to begin treatment with allopurinol in a hospital and combine the drug with nonsteroidal anti-inflammatory drugs or colchicine (1.5 mg/day) for 7–10 days. In the first weeks of treatment of urate nephrolithiasis with allopurinol, it is advisable to combine it with drugs that increase the solubility of urate in urine (magurlit, uralit, potassium bicarbonate, diacarb). In chronic tubulointerstitial nephritis, the dose of allopurinol is reduced as glomerular filtration rate decreases, and in cases of severe chronic renal failure (serum creatinine > 500 μmol/l), it is contraindicated. Allopurinol enhances the effect of indirect anticoagulants and aggravates the toxic effect of azathioprine on the bone marrow. If hyperuricemia (gout) is detected in a recipient after transplantation, it is necessary to reduce the dose of cyclosporine and saluretics. If there is no effect, replace azathioprine with mycophenolate mofetil and only then add allopurinol [10].
Uricosuric drugs correct hyperuricemia by increasing uric acid excretion in the urine. Used for asymptomatic hyperuricemia, gouty chronic tubulointerstitial nephritis. Contraindicated in hyperuricosuria, urate nephrolithiasis, and chronic renal failure. The most commonly used are probenecid (initial dose 0.5 g/day), sulfinpyrazone (0.1 g/day), and benzobromarone (0.1 g/day). A combination of allopurinol with benzobromarone or sulfinpyrazone is possible. Losartan and other II receptor blockers also have a uricosuric effect.
Citrate mixtures (uralit, magurlit, blemaren) correct metabolic acidosis, increase urine pH to 6.5–7 and thereby dissolve small urate stones. Indicated for urate nephrolithiasis. Uralite or Magurlit is taken before meals 3-4 times a day in a daily dose of 6-10 g. During treatment, constant monitoring of urine pH is necessary, since its sharp alkalization can lead to crystallization of phosphates. Citrate mixtures are contraindicated in chronic renal failure, with active pyelonephritis, and should be used with caution in hypertension (contain a lot of sodium). Citrate mixtures are not effective for large stones when extracorporeal lithotripsy or pyelolithotomy is indicated.
The objectives of antihypertensive therapy for gouty nephropathy include ensuring nephroprotective and cardioprotective effects. Drugs that retain uric acid (thiazide diuretics) and aggravate hyperlipidemia (non-selective β-blockers) should not be used. The drugs of choice are ACE inhibitors, A II receptor blockers, calcium antagonists, and selective β-blockers.
Statins (lovastatin, fluvastatin, pravastatin) are used in patients with gout with low-density lipoprotein cholesterol levels > 130 mg%. III generation statins (atorvastatin) have an independent hypouricemic effect [11].
The most effective treatment for gouty nephropathy is a combination of ACE inhibitors with A II receptor blockers, statins and allopurinol. With this combination, hypouricemic, antiproteinuric, hypolipidemic and hypotensive effects are enhanced with restoration of the circadian rhythm of blood pressure and slowing down the remodeling of the left ventricular myocardium, the risk of developing metabolic syndrome and diabetes mellitus is reduced, and the concentration of C-reactive protein in the blood decreases. As a result, the risk of developing acute myocardial infarction, acute cerebrovascular accidents and outcome in chronic renal failure is reduced.
Literature
- Bunchuk N.V. Gout // Rheumatic diseases / ed. V. A. Nasonova and N. V. Bunchuk. M., 1997. pp. 363–374.
- Mukhin N. A., Balkarov I. M. Gouty kidney // Nephrology / ed. I. E. Tareeva. M., 2000. pp. 422–429.
- Stamatelou KK , Francis ME, Jones CA Time trends is the reported prevalence of kidney stones in the US// Kidney Int. 2003; 63:1817–1823.
- Bingham C., Ellard S. et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1 beta gene mutation // Kidney Int. 2003; 63:1645–1651.
- Kang DH, Nakagawa T., Feng L. A role of uric acid in the progression of renal disease // J. Amer. Soc. Nephrol. 2002; 13:2888–2897.
- Nikolaev A. Yu. Disorders of purine metabolism in alcoholism // Alcohol disease / ed. V. S. Moiseeva. M., 1990. pp. 95–99.
- Karumanchi SA, Maynard SE, Stillman IE Preeclampsia: a renal perspective // Kidney Int. 2005; 67:2101–2113.
- Ohno T., Hosoya T., Gomi H. Serum uric acid and renal prognosis in IgA nephropathy // Nephron - 2001; 87:333–339.
- Munter P., He J., Vupputuri S. Blood lead and CKD in the general US population: results from NHANES III. Kidney Int. 2003; 63:1044–1050.
- Perez-Ruiz F., Gomez-Ullate P., Amenabar J. Long-term effectiveness of hyperuricaemia treatment of renal transplant patients // Nephrol. Dial. Transpl. 2003; 18: 603–606.
- Athyros VG, Elisaf M., Papageorgiou AA Effect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the GREck Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study // Amer. J. Kidney Dis. 2004; 43:589–599.
A. Yu. Nikolaev , Doctor of Medical Sciences, Professor Yu. S. Milovanov , Candidate of Medical Sciences, Associate Professor of MMA named after. I. M. Sechenova, Moscow
Dysmetabolic nephropathy
This term combines kidney damage caused by severe metabolic damage: primary and secondary hyperuraturia and hyperoxalaturia, damage due to diabetes mellitus, hypoxia, shock, hypokalemia, hypercalcemia.
A characteristic symptom is damage to the interstitial tissue of the kidneys by the deposition of salts in it and the further development of cellular infiltrates, fibrosis, disruption of the trophism of the kidney tubules, focal atrophy, dilatation and regeneration in them.
Oxalate nephropathy
– this tubulopathy is one of the forms of the anomaly. Hyperoxalaturia can be primary (a rare hereditary fermentopathy with primary synthesis of oxalates) or secondary (hereditary polygenic family instability of membranes, against which the provoking role is played by deficiency of vitamins B6, A, E, hypoxia, hypercalcemia, for example, with hypervitaminosis of vitamin D).
Clinic.
Oxanosis manifests itself at an early age. The child experiences recurrent pain in the joints and abdomen. The most important is kidney damage due to the development of nephrocalcinosis, interstitial nephritis, pyelonephritis, with a typical clinical picture.
Treatment.
The basis of therapy is nutritional correction, drinking regimen and drug therapy.
The diet is hypooxalate or the so-called cabbage-potato diet. Potatoes contain a moderate amount of oxalates, which are not absorbed from the gastrointestinal tract, because the significant amount of calcium present in potatoes keeps oxalates in undissolved form. This causes almost complete excretion of oxalates in feces. Oxalogenic foods are excluded: leafy vegetables (lettuce, spinach, rhubarb), tomatoes, carrots, strong tea, coffee, cocoa, chocolate, strong broths. You should also limit meat (beef, chicken), liver, fish (cod), foods containing large quantities of ascorbic acid: apples, radishes, tomatoes, currants. It is better to eat meat boiled. The diet includes fresh lard, vegetable and butter, sour cream, unsweetened fruits: pear, prunes, quince, dried apricots, white bread.
A hypooxalate diet is prescribed for 2-3 weeks with an interval of 2-3 weeks, during which the child is prescribed table No. 5, a large amount of liquid is prescribed, especially in the evening hours, when the urine is most concentrated, which creates conditions for the crystallization of various salts in the kidneys and urinary tract. bubble.
Decoctions of lingonberries, flaxseed, pear bark, cherries, and mineral waters (Smirnovskaya, Slavyanovskaya, Essentuki, Sairme, Naftuzi) are used at a rate of 5 ml/kg single dose, between meals and at night. Change mineral water every 3-4 weeks. A necessary condition for successful treatment is compliance with the regime of forced urination - every 1.5 - 2 hours or forced diuresis (taking diuretics).
Among the medications, the use of salts of a number of metals that inhibit the formation of urinary stones is indicated: magnesium oxide at a dose of 0.15-0.2 g per day once in the morning for 3-4 weeks; vitamins B6, A, E, which provide an anti-inflammatory effect in the kidneys. Vitamin A in a dose of 5-10 mg 1 time per day after meals (1 drop per year of life) for 1 month. At the same time, vitamin E (up to 10 drops of a 5% oil solution, 1 r per day). Vitamin courses are carried out 3-4 times a year. After vitamin therapy, diphospholates are used - 2% xidifon and 15% dimephosphon, which stabilize membranes and calcium metabolism. They are prescribed 1 tsp. – 1 tbsp. – 3 times a day for 1 month, 2 – 3 times a year.
Prevent the formation of stones consisting of calcium oxalate, urates, uric acid and promote their dissolution with preparations containing a mixture of K, Mg, Na, citric acid and vitamins. These drugs are: Magurlit (Hungary), Blemaren (Germany), Solimok, Soluran, Uralit-I. When using them, the pH of the urine is controlled so as not to lead to alkalization with the formation of phosphates.
Urate nephropathy. Classic diseases inherited in a recessive X-linked manner with increased urate synthesis are gout and Lesch-Nyhan syndrome.
Gout manifests itself in children as a neuro-arthritic diathesis.
Lesch-Nyham syndrome occurs only in boys. It is based on a defect in the enzyme hypoxanthine guanine phosphoribofiltransferase. Already in the first months of life, a delay in psychomotor development is detected, and then hyperreflexia, clonus of the feet and spasticity of the limbs, and choreoathetoid movements are added. Children are prone to self-injurious behavior (biting lips, tongue, fingers, pulling out hair, etc.) and are aggressive towards others.
The diagnosis is made based on clinical manifestations and high levels of uric acid in the blood (10 times or more above normal).
Secondary urate nephropathy occurs when a large number of nucleated cells disintegrate, resulting in the formation of a significant number of purines and pyrimidines, the end product of which is uric acid.
Treatment: if there is a violation of purine metabolism, it is aimed at reducing the flow of urates into the kidneys. First of all, diet. The maximum amount of purines is contained in the following products and therefore these products are excluded from the diet: herring, sprats, sardines, cod, rabbit meat, chicken, lamb, meat broths, liver, kidneys, brains, spinach, Brussels sprouts, cocoa, nuts, chocolate, beans, lentils, peas, beans, smoked ham. The minimum amount of purines is found in the following foods: cauliflower, radishes, cereals (rice), potatoes, fruits, eggs, milk. And therefore these products are included in the hypourate diet.
The second important point in treatment is ensuring a high-fluid drinking regime with the intake of alkalizing mineral waters, which slows down the sedimentation of urates. It is advisable to add lemon juice to your drink.
Herbal medicine includes decoctions of oats, barley and other cereals with hemp, bran (rye flour or dry wheat flour), as well as decoctions of herbs that have an anti-sclerotic effect (rose hips, immortelle, rose petals, lingonberry leaves, buckwheat grass. Oregano, eucalyptus, tansy ); anti-inflammatory effect (bearberry, St. John's wort, chamomile, lingonberry leaf, juniper berries, oregano, sage, calendula, eucalyptus, poplar buds). Diuretic effect (birch leaves and buds, lingonberry knotweed leaf, licorice root. Madder, mint leaves, red rowan fruits, parsley, bearberry).
Ready-made dosage forms are available: Avisan. Phytolysin, madder extract, olimethine, urolesan.
Due to frequent side effects, the use of allopurinol in pediatric practice is limited.
Dispensary observation of children with nephropathies is carried out for 3 years, with monitoring of urine flow and the functional state of the kidneys. Nephrologist examinations 1 r. in year. Ultrasound of the kidneys 2 r. in year. Urine according to Zimnitsky 1 rub. in year. Examination by a pediatrician quarterly.
The material was prepared by I. Yu. Sidalieva, a local pediatrician at the State Budgetary Healthcare Institution JSC “Child Hospital No. 4”