general description
Diabetic nephropathy (DN) is a lesion of the glomerular apparatus of the kidneys due to diabetic microangiopathy.
The term “DN” often refers to a specific kidney disorder—diabetic glomerulosclerosis (DH). The prevalence of diabetic nephropathy reaches 40–50% in patients with type 1 diabetes mellitus and 15–30% in patients with type 2 diabetes mellitus; occurs more often in women. Diabetic nephropathy is the leading cause of high morbidity and mortality in patients with diabetes.
Damage to the renal vascular system is caused by hyperglycemia and high blood pressure. Under these conditions, the kidneys can no longer properly perform the filtration function, so substances that are normally retained by the kidneys and remain in the body (for example, protein) are detected in the urine.
There are 3 stages of diabetic nephropathy:
- Stage of microalbuminuria.
- Stage of proteinuria with preserved nitrogen excretion function of the kidneys.
- Stage of chronic renal failure.
Pathogenetic mechanisms of nephropathy formation in type 1 diabetes mellitus
In recent years, there has been an increase in the incidence of secondary kidney diseases in children associated with metabolic disorders and other endocrine diseases, which have a progressive course with the development of chronic kidney disease (CKD) and disability of patients already in childhood [1].
Diabetes mellitus (DM) is one of the common childhood diseases. In Russia, 2.3 million people with diabetes are registered, of which 14 thousand are children and 10 thousand are teenagers. Severe specific complications of diabetes lead to decreased ability to work, worsening the prognosis of the patient’s life, and have identified type 1 diabetes as the most important medical, social and economic problem of modern society [2]. One of the most serious complications of type 1 diabetes is kidney damage.
An alarming fact is the underdiagnosis of diabetic nephropathy (DN) in pediatric practice [3].
Modern screening tests make it possible to detect DN only from the 1st clinical stage - the stage of microalbuminuria (MAU), while missing the initial structural and functional disorders that develop long before the increase in albumin excretion [4].
Tubulointerstitial fibrosis is an important mechanism of loss of renal function in diabetes, which precedes glomerulosclerosis [5].
Damage to the tubular apparatus occurs in the early stages of the development of nephropathy, even before the appearance of obvious clinical signs, and dysfunction of the endothelium develops long before the occurrence of structural changes in the kidneys. Moreover, changes in the parameters of endothelium-dependent vasodilation occur in parallel with a decrease in glomerular filtration rate and correlate with the levels of biomolecular markers of inflammation [6].
Endothelial dysfunction (ED) is considered as a central link in the pathogenesis of many chronic diseases [7].
Endothelial dysfunction is an integral marker of target organ damage in arterial hypertension (AH), diabetes and metabolic syndrome, primarily the kidneys [8, 9].
Currently, endothelial dysfunction is understood as an imbalance between the formation of vasodilating, atrombogenic, antiproliferative factors, on the one hand, and vasoconstrictive, prothrombotic and proliferative substances synthesized by the endothelium, on the other [10].
The main factors that activate endothelial cells [11]:
- change in blood flow speed;
- platelet factors;
- hormones and mediators (catecholamines, acetylcholine, endothelin, bradykinin, angiotensin II (AT II));
- hypoxia.
Markers of ED include decreased endothelial synthesis of nitric oxide (NO), increased levels of endothelin-1, circulating von Willebrand factor, plasminogen activator inhibitor, homocysteine, thrombomodulin, soluble vascular intercellular adhesion molecule B1, C-reactive protein, microalbuminuria, and others [12].
Endothelial dysfunction precedes the development of clinical manifestations of diseases, including kidney damage, therefore assessment of endothelial functions is of great diagnostic and prognostic importance [7].
In type 1 diabetes, hyperglycemia induces non-enzymatic protein glycation, oxidative stress, activates protein kinase C, mitogen-activating protein kinase, the action of growth factors, vasoactive factors, cytokines that cause kidney damage at the cellular level. This leads to the development of renal hypertrophy and accumulation of extracellular matrix, which precedes such irreversible changes as glomerulosclerosis and tubulointerstitial fibrosis [6]. One of the main mechanisms for the development and progression of DN is considered to be intraglomerular hyperfiltration and hypertension, which is the leading hemodynamic factor in the progression of DN [13]. This mechanism is activated by chronic hyperglycemia, causing first functional and then structural changes in the kidneys, which occur latently and lead to the appearance of MAU. The main triggers for the appearance of MAU, in addition to hyperglycemia, are endothelial dysfunction and glomerular hyperfiltration. An increase in intraglomerular pressure occurs due to narrowing of the efferent arteriole as a result of a direct effect on the vascular endothelium or hyperactivation of the sympathetic nervous system. As a result of these changes, there is an increase in filtration processes—hyperfiltration—and an increase in the flow of albumin into primary urine [14]. Microalbuminuria is a proven highly sensitive marker of prognostically unfavorable kidney damage, and also reflects the presence of ED. The appearance of MAU indicates the presence of sclerosis of at least 20–25% of nephrons, and progression to the stage of proteinuria indicates the loss of 50–70% of the glomeruli, which indicates irreversible kidney damage, which sharply reduces the effectiveness of the therapy, and a progressive decrease in the filtration function of the kidneys becomes inevitable [15].
Prolonged exposure to hyperglycemia and powerful hemodynamic shock (“shear stress”) initiates mechanical irritation of the adjacent structures of the glomerulus, which promotes hyperproduction of collagen and its accumulation in the mesangium, initial sclerotic processes, disruption of the architectonics and permeability of the glomerular basement membrane [16].
A hemodynamic shock can itself lead to an increase in the expression by endothelial cells of some vasoactive molecules with a predominantly vasoconstrictor effect - angiotensin II, endothelin-1, adhesion molecules (VCAM-1), components of the endothelium-dependent hemostasis - PAI-1 (plasminogen activator inhibitor type 1) [17]. Along with this, the activity of collagenolytic enzymes in the kidneys decreases. As a result, excessive accumulation of collagen occurs, which becomes a key link in the development of diabetic nephrosclerosis.
Intraglomerular hyperfiltration triggers a compensatory cascade of activation of the renin-angiotensin-aldosterone system (RAAS), which further contributes to the progression of functional and hemodynamic disorders in the kidneys [18].
The mechanisms of the pathogenic action of angiotensin II (AII) in diabetes are due not only to its powerful vasoconstrictor effect, but also to proliferative, prooxidant and prothrombogenic activity. In the kidneys, AII causes intraglomerular hypertension, promotes sclerosis and fibrosis of renal tissue indirectly through the release of cytokines and growth factors [6].
ATII, including its local renal pool, stimulates the proliferation of mesangial cells and their production of collagens, chemotaxis factors and transforming growth factor β1, which contribute to the increase in macrophage infiltration, tubulointerstitial inflammation and fibrosis and, ultimately, the progression of glomerulosclerosis. In addition, ATII directly increases the permeability of the glomerular basement membrane, thereby contributing to the increase in proteinuria. By causing spasm predominantly of the efferent arteriole, ATII causes an increase in intraglomerular pressure and an increase in the gradient of renal transcapillary pressure. Spasm of the glomerular arteries can lead to redistribution of blood in the kidney: its shunting into the vessels of the renal pyramids increases, which leads to partial ischemia of the structures of the renal cortex [19].
Hypertension plays a key role in the development and progression of DN, as well as in the development of macrovascular pathology. As DN progresses, the role of metabolic factors decreases and the role of hemodynamic factors (hypertension, intraglomerular hypertension) increases [6].
Recent studies have established a direct damaging effect on the structure of renal tissue and the endothelium of renal vessels of hypercholesterolemia, atherogenic lipid fractions, lipid mediators (prostaglandins), cytokines, such as tumor necrosis factor alpha (TNF-α), interleukins-1, 6, 8 [ 19, 20].
TNF-α, being one of the key pro-inflammatory factors, stimulates the activation of the nuclear transcription factor with a subsequent increase in the synthesis of endothelin-1 in the mesangial cells of the kidneys and activation of the processes of proliferation and sclerosis in the kidney tissue [21].
It is also known that the mesangial cells of the renal glomeruli have receptors for low-density lipoproteins, which, under conditions of hyperlipidemia, contributes to their accumulation, including oxidized forms. Oxidized forms cause damage to cell wall proteins. As a result of activation of proteolytic systems, the mesangium is infiltrated by macrophages and mononuclear cells, the growth factors and cytokines released in this process cause an increase in the production of mesangial matrix components and glomerular basement membrane substance (GBM). Lipoproteins deposited in the renal tissue bind negatively charged glycosaminoglycans of the BMK, neutralizing the charge and increasing its permeability. There is a decrease in the activity of NO synthase, a decrease in the synthesis of prostacyclin, which leads to an increase in the production of vasoconstrictor substances by the vascular endothelium (endothelin-1, thromboxane A2), and the vasomotor function of the endothelium is disrupted [22, 23].
The role of lipids in the biology of podocytes has been established. After exposure to various pathogenic factors (metabolic, toxic, hemodynamic), podocytes undergo structural and functional changes, which are called podocytopathies. Signs of podocytopathy are smoothing of the podocyte stalks with impaired permeability of the slit-like diaphragm, hypertrophy, apoptosis, detachment of podocytes from the BM with their desquamation into the urinary space and the appearance in the urine of both whole cells (podocyturia) and its structural proteins (nephrin, podocin, etc.) , decrease in the number of podocytes in the glomerulus (podocytopenia). It has now been established that the phenomenon of smoothing of the pedunculate processes is a nonspecific reaction of the epithelial cell to the action of a pathogenic factor. Nephrin is a transmembrane protein involved in binding to the actin cytoskeleton of podocytes, on the other hand, through the interaction of extracellular domains with each other - in the formation of the interpodocyte slit diaphragm. If the pores are intact, large protein molecules, for example, serum albumin, cannot pass through the barrier [24–26]. Increasing protein filtration as a result of these injuries can, in turn, lead to excessive secretion by the proximal tubular epithelium of the specific renal fibrosis factor MCP-1, the expression of which is induced by hyperglycemia. MCP-1 causes worsening of inflammation and fibrosis in the renal tubules and interstitium. Increased excretion of nephrin in the urine suggests podocytopathy [24, 25].
In the pathogenesis of ED in diabetes, factors of progression of DN (hyperglycemia, dyslipidemia, intraglomerular hypertension and cytokines) play a role, which cause deep damage to the vascular endothelium and lead to the release of “mediators” of damage (angiotensins, endothelins, cytokines, C-reactive protein, TNF, etc. .), contributing to the initiation and progression of diabetic microangiopathies [27].
However, the development of DN does not always depend on the level of compensation of carbohydrate metabolism and the duration of diabetes, which suggests the existence of genetic factors that determine one or another degree of sensitivity of the renal vessels to the effects of metabolic and hemodynamic disorders [28].
Currently, the main approach to studying genetic predisposition to diabetic angiopathy is the use of polymorphic markers linked to various candidate genes (genes whose expression products may be directly or indirectly involved in the development of the pathology) [29, 30].
It is known that in the pathogenesis of DN the most important role is played by the balance between the activity of the pressor renin-angiotensin system [31, 32] and the depressor system of NO synthesis [33].
Thus, the genetic determinants of the RAAS are of interest. Among the latter, much attention has been paid to the angiotensin I converting enzyme gene, including its insertion-deletion polymorphic marker. Under the influence of this enzyme, the formation of angiotensin II, the most active vasoconstrictor peptide, and the degradation of bradykinin, an important vasodilator factor, occur [34].
Hereditary factors that directly or indirectly form a genetic predisposition to this pathology include the endothelial NO synthase (eNOS) gene. Some authors consider the potential role of this gene as a risk factor for the development of atherosclerosis and diseases leading to disruption of the normal production of nitric oxide [35].
Nevertheless, the leading role in the progression of DN to terminal stages is played by hemodynamic factors (arterial hypertension), while the role of metabolic and genetic factors in the progression of DN is weakening [28].
Timely diagnosis of DN in the early stages in patients with diabetes is a very important task. It is necessary to search for available markers of the early stage of kidney damage and create an algorithm for forming risk groups for diabetic nephropathy [36].
For patients who developed diabetes in childhood, this problem is most relevant, since in children type 1 diabetes is labile, with frequent decompensations, which can contribute to the earlier development of complications, including DN [37].
It has been established that kidney pathology in type 1 diabetes in children is formed against the background of disturbances in carbohydrate and lipid metabolism, changes in intrarenal hemodynamics, hyperleptinemia and infection of the urinary system with pathogenic uroflora [38].
In the development of tubulointerstitial kidney damage, the role of complex processes of intercellular interactions, which are activated under the influence of immune and non-immune factors, has been determined [6].
Thus, a comprehensive clinical and laboratory examination of patients with type 1 diabetes mellitus using molecular biological technologies is necessary to prevent the development of DN along with the assessment of metabolic indicators (hyperglycemia, dyslipidemia) and hemodynamic (arterial hypertension), markers of ED, as well as genetic risk factors DN [28].
Literature
- Vyalkova AA Chronic kidney disease in pediatric nephrology / Materials of the International School and Scientific and Practical Conference on Pediatric Nephrology “Current Problems of Pediatric Nephrology”. Orenburg, 2010. pp. 63–75.
- Shestakova M.V., Chugunova L.A., Shakhmalova M.Sh., Dedov I.I. Diabetic nephropathy: advances in diagnosis, prevention and treatment // Diabetes mellitus. 2005. No. 3. pp. 22–25.
- Shestakova M.V. Diabetic nephropathy: fatal or preventable complication? // Rus. honey. magazine. 2007. No. 3.
- Dedov I. I., Shestakova M. V. Diabetes mellitus and chronic kidney disease. M.: Medical Information Agency LLC, 2009. P. 482.
- Shamkhalova M. Sh., Kurumova K. O., Klefortova I. I., Sitkin I. I., Ilyin A. V., Arbuzova M. I., Goncharov N. P., Katsiya G. V., Aleksandrov A A., Kukharenko S.S., Shestakova M.V., Dedov I.I. Factors in the development of tubulointerstitial kidney damage in patients with diabetes mellitus // Diabetes mellitus. 2010. No. 3. pp. 134–141.
- Shestakova M. V., Shamkhalova M. Sh., Jarek-Martynova I. Ya., Klefortova I. I., Sukhareva O. Yu., Vikulova O. K., Zaitseva N. V., Martynov S. A., Kvaratskhelia M.V., Tarasov E.V., Trubitsyna N.P. Diabetes mellitus and chronic kidney disease: achievements, unresolved problems and treatment prospects // Diabetes mellitus. 2011. No. 1. pp. 81–88.
- Melnikova Yu. S., Makarova T. P. Endothelial dysfunction as a central link in the pathogenesis of chronic diseases // Kazan Medical Journal. 2015. T. 96, no. 4. pp. 659–665.
- Kochueva M.N., Gavrilyuk V.A. Neuroendocrine mechanisms of nephropathy development in patients with essential arterial hypertension with obesity // Skhidnoevropeyskiy journal of internal and family medicine. 2014. No. 1. P. 43–50.
- Sharma K. Adiponectin regulates albuminuria and podocyte function in mice // J. Clin. Invest. 2008. Vol. 118. R. 1645–1656.
- Group . Markers of endothelial dysfunction. In the book: Group Catalog. M., 2005. pp. 49–50.
- Dzau V., Bernstein K., Celermaier D. et al. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data // Am J Cardiol. 200.Vol. 88 (suppl. L). R. 1–20.
- Konyukh E. A., Paramonova N. S. Clinical features of the course of acute and chronic glomerulonephritis in children with endothelial dysfunction // Journal of GrSMU. 2010, no. 2. pp. 149–151.
- Arutyunov G.P., Oganezova L.G. Frequently asked questions about glomerular filtration rate // Clinical nephrology. 2009. No. 3. P. 35–42.
- McCullough PA, Li S, Jurkovitz CT et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality // Am Heart J. 2008. Vol. 156. R. 277–283.
- Shestakova M.V. Modern concept of “chronic kidney disease”: diagnostic methods, clinical significance // Diabetes mellitus. 2008. No. 2. P. 4–7.
- Brenner BM, Hostetter T., Humes HD Molecular basis of proteinuria of glomerular origin // N. Engl. J. Med. 1978. No. 298. P. 826–833
- Belyaeva O. D., Bazhenova E. A., Berezina A. V. et al. Leptin level, genotype distribution and occurrence of alleles A19 G polymorphism of the leptin gene in patients with abdominal obesity // Art. hypertension. 2009, no. 4. pp. 440–444.
- Garrido AM, Griendling KK NADPH oxidases and angiotensin II receptor signaling // Molecular and Cellular Endocrinology. 2009. Vol. 302, No. 2. R. 148–158.
- Wiecek A., Kokot F., Chudek J., Adamczak M. The adipose tissue is a novel endocrine organ of interest to the nephrologists // Nephrol Dial Transplant. 2002. No. 17. R. 191–195.
- Nikolaeva S. N., Lebedeva E. N., Vyalkova A. A. et al. Clinical assessment of leptin and insulin levels in the blood in obese children // Sovrem. question pediatrics. 2007. pp. 485–486.
- Sharma K., McCue P., Dunn S. Diabetic kidney disease in the db/db mouse // Am J Renal Physiol. 2003. Vol. 284. R. 1138–1144.
- Kryachkova A. A., Savelyeva S. A., Gallyamov M. G. et al. The role of obesity in kidney damage in metabolic syndrome // Nephrology and Dialysis. 2010. T. 12, No. 1. P. 34–38.
- Nanchikeeva M. L., Kozlovskaya L. V., Fomin V. V. et al. Endothelial dysfunction and remodeling of intrarenal vessels as the basis for the formation of hypertensive nephropathy // Ultrasound and functional diagnostics. 2009. No. 5. pp. 84–94.
- Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes // Am J Physiol Renal Physiol. 2005. Vol. 288, No. 1. R. 48–57.
- Fornoni A., Merscher S., Kopp JB Lipid biology of the podocyte - new perspectives offer new opportunities // Nat Rev Nephrol. 2014, Vol. 10, No. 7. R. 379–88.
- Bobkova I.N., Shestakova M.V., Shchukina A.A. Damage to podocytes in diabetes mellitus // Diabetes mellitus. 2014. No. 3. P. 39.
- Dedov I. I., Shestakova M. V. Diabetic nephropathy. M.: Universum Publishing, 2000. 239 p.
- Vikulova O. K. Clinical, laboratory and genetic factors in the development and progression of diabetic nephropathy in patients with type 1 diabetes mellitus. Author's abstract. dis. ... Ph.D. M., 2003. P. 123.
- Kondratiev Ya. Yu., Shestakova M. V., Chugunova L. A. et al. Strategy for searching for markers of genetic predisposition to vascular complications of diabetes mellitus using the example of diabetic nephropathy // Diabetes mellitus. 1998. No. 1. pp. 22–25.
- Abbut ZA, Wilson AC, Cosgrove NM et al. Angiotensin-converting enzyme gene polymorphism in systemic hypertension // Am J Cardiol. 1998. Vol. 81. R. 244–246.
- Apperloo AJ, de Zeeuw D., de Jong PE Short-term antiproteinuric responses to antihypertensive treatment predicts long-term GFR decline in patients with non-diabetic renal disease // Kidney Int. 1994. Vol. 45, Suppl. 45, p. S174-S178.
- Jones SC, Thomas T.N., Marshall SM Abnormal regulation of cell membrane fluidity in diabetic nephropathy // Diabetologia. 1998. Vol. 41, Iss. 3, r. 337–342.
- Shestakova M.V., Chugunova JI. A., Shamkhalova M. Sh., Nosikov V. V. Genetic factors in the development of diabetic nephropathy. M., 2002.
- Kuixing Yi. Z. et al. Angiotensin II type 2 receptor gene polymorphisms and essential hypertension // Acta Pharmacol Sin. 2003. Vol. 24, No. 11. P. 1089–1090.
- Bondar I. A., Klimontov V. V., Porshennikov I. A. Nitric oxide and diabetic angiopathies // Diabetes mellitus. 1999. No. 4. pp. 11–14.
- Mukhin N.A., Arutyunov G.P., Fomin V.V. Albuminuria is a marker of kidney damage and the risk of cardiovascular complications // Nephrology. 2009. No. 1. P. 12–17.
- Dedov I. I., Peterkova V. A., Kuraeva T. L. et al. Complications of diabetes mellitus in children and adolescents. M.: GU ENTS RAMS, 2003. 96 p.
- Vyalkova A. A., Savelyeva E. V., Kulagina E. P., Belova M. A. Early diagnosis of kidney damage in children with type 1 diabetes // Pediatrician (conference materials). 2021. T. 7, issue. 2, p. 187–188.
L. V. Kutsenko I. V. Zorin1, Doctor of Medical Sciences, Professor A. A. Vyalkova, Doctor of Medical Sciences, Professor
Federal State Budgetary Educational Institution of Higher Education Org State Medical University of the Ministry of Health of the Russian Federation, Orenburg
1 Contact information
Pathogenetic mechanisms of the formation of nephropathy in type 1 diabetes mellitus L. V. Kutsenko, I. V. Zorin, A. A. Vyalkova For citation: Attending physician No. 6/2018; Page numbers in the issue: 58-61 Tags: diabetes mellitus, complications, kidney damage, early stage markers
Clinical picture
- Proteinuria (stage 1 - a small amount of protein in the urine, stages 2 and 3 - a large amount of protein in the urine).
- Arterial hypertension (stages 1, 2 and 3).
- Nocturia (frequent urination at night) (stage 3).
- Polyuria (excretion of 2–4 liters of urine per day) (stage 3).
- Shortness of breath, pain in the heart area (stage 3).
- Dryness and bitterness in the mouth, lack of appetite, pain and heaviness in the epigastric region, loose stools.
- Nose and gastrointestinal bleeding, skin hemorrhages (stage 3).
- General weakness, nausea, vomiting, skin itching, muscle twitching (stage 3).
Treatment of diabetic nephropathy
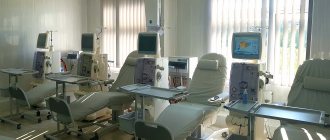
Treatment begins with normalizing blood sugar levels. For arterial hypertension - normalization of blood pressure with ACE inhibitors, which, when taken continuously, slow down the progression of nephropathy. The nephroprotective effect is enhanced by the addition of Verapamil. Normalization of cholesterol levels is shown. Restriction in the diet of animal protein is required. At stage 3, correction of water and electrolyte metabolism and methods of renal replacement therapy are necessary: hemodialysis, peritoneal dialysis, kidney transplantation.
Essential drugs
There are contraindications. Specialist consultation is required.
- Enalapril (ACE inhibitor, antihypertensive, nephroprotector). Dosage regimen: take 2.5-10 mg orally 2 times a day, constantly.
- Verapamil (antiarrhythmic, antianginal and antihypertensive agent, nephroprotector). Dosage regimen: the drug is taken orally, before meals, with a small amount of water. To prevent attacks of angina pectoris, arrhythmia and in the treatment of arterial hypertension, the drug is prescribed to adults at an initial dose of 40-80 mg 3-4 times a day. If necessary, increase the single dose to 120-160 mg. The maximum daily dose of the drug is 480 mg. The duration of treatment is determined individually depending on the patient’s condition, severity, characteristics of the course of the disease and the effectiveness of therapy.
- Valsartan (antihypertensive drug, nephroprotector). Dosage regimen: take 80-160 mg orally once a day, continuously.
- Atorvastatin (lipid-lowering drug). Dosage regimen: take 5-20 mg orally once a day, the duration of therapy is determined individually.
Prevention
For diabetic patients, prevention of nephropathy should include several main points:
- maintaining a safe blood sugar level (regulate physical activity, avoid stress and constantly measure glucose levels);
- proper nutrition (diet with a reduced percentage of proteins and carbohydrates, giving up cigarettes and alcohol);
- control over the ratio of lipids in the blood;
- monitoring blood pressure levels (if it rises above 140/90 mm Hg, urgent action must be taken).
All preventive measures must be coordinated with your doctor. The therapeutic diet must also be carried out under the strict supervision of an endocrinologist and nephrologist.
Incidence (per 100,000 people)
| Men | Women | |||||||||||||
| Age, years | 0-1 | 1-3 | 3-14 | 14-25 | 25-40 | 40-60 | 60 + | 0-1 | 1-3 | 3-14 | 14-25 | 25-40 | 40-60 | 60 + |
| Number of sick people | 0.01 | 0.05 | 0.1 | 5 | 5 | 10 | 15 | 0.01 | 0.05 | 0.1 | 5 | 5 | 10 | 15 |