Tell me, how many of us, while consuming a fragrant piece of fried meat or a fresh cutlet, thought about whether the body has enough acid and alkali to process it all? The acid-base balance, as scientists have found out in our century, is the main thing that determines how healthy, alert and cheerful a person will be. On the packaging of almost any food product you can find information about how much protein, fat and carbohydrates it contains and what the energy value of 100 g of this food is.
American scientists at the beginning of the 21st century made a genuine discovery when they discovered that any product has another fundamental indicator that is critical for our health - the acid load of food. It consists of the ratio of components in food that, during metabolism, form either an acid or an alkali.
The acid load (AL) is measured using the acid minus alkali principle.
When components that form sulfuric acid (sulfur-containing amino acids in proteins) or organic acids (fats, carbohydrates) predominate in food, then KH has a positive value.
If the food contains more components that form alkali (organic salts of magnesium, calcium, potassium), then KN is a negative value.
Based on computer analysis, these scientists compiled a table of the acid load of basic foods.
Acid load of staple foods (in milliequivalents per 240 kilocalories)
| Sour foods | Neutral products | Alkaline products |
| Meat = 67.9 | Legumes- = -0.8 | Leafy greens= -59.1 |
| Cereals = 13.8 | Nuts= 0.1 | Vegetables= -46.5 |
| Cheese = 4.2 | Roots= -26.4 | |
| Milk and yogurt = 2.8 | Tubers= -10.6 | |
| Eggs = 2.5 | Fruit= -5.8 |
Source: American Journal of Clinical Nutrition. 2002.76(6) 1308-1316
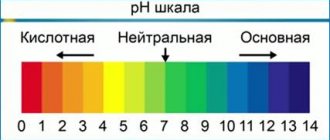
pH, or acid-base balance indicator
It is a measure of the relative concentration of hydrogen (H+) and hydroxyl (OH-) ions in a liquid system and is expressed on a scale from 0 (complete saturation with hydrogen ions H+) to 14 (complete saturation with hydroxyl ions OH-), distilled water is considered neutral with pH 7.0.
An increase in the concentration of positive hydrogen ions (H+) in any body fluid causes a shift in pH values towards zero and is called an acid shift.
An increase in the concentration of hydroxyl ions OH causes a shift in pH values towards 14 and is called an alkaline shift.
Arterial blood pH = 7.35-7.45 Venous blood pH = 7.26-7.36 Lymph pH = 7.35-7.4 Intercellular fluid pH = 7.26-7.38 Intra-articular fluid pH = 7.3
Acid-base balance is an indicator of health.
Man is a single biological system. Every minute, every second, billions of cells are born and die in us, they are constantly renewed. All these processes are absolutely impossible without oxygen, water and hydrogen. Our body lives and functions properly only in a slightly alkaline environment. The pH of the main body fluid - blood - is 7.43 (+/- 0.02). This indicator is the same for all people on Earth. pH is the body’s hydrogen indicator, it is the regulator of all systems and organs, the leading constant of life! Blood pH The pH level of your blood is maintained by the body in a narrow range of 7.35-7.45. The pH level of the blood must always remain at a safe level, so the body uses the above-mentioned organs and tissues to maintain it. Kidney pH The pH level of the kidneys is affected by both water and food, as well as metabolic processes in the body. Acidic foods (such as meat products, dairy products, etc.) and drinks (sweetened drinks, alcoholic drinks, coffee, etc.) lead to low pH levels in the kidneys because the body eliminates excess acidity through urine. The lower the urine pH level, the harder the kidneys have to work. Therefore, the acid load placed on the kidneys from such foods and drinks is called potential acid-renal load. The benefit that water brings to the kidneys is that it increases the pH level of the urine, which will reduce the acid load that the kidneys will need to get rid of. Increasing urine pH raises the body's pH and rids the kidneys of acidic toxins. Stomach pH An empty stomach contains no more than a teaspoon of stomach acid produced in the last meal. The stomach produces acid when it is needed. The stomach does not produce acid when you drink water. The most useful thing is to drink water on an empty stomach - the pH thus increases to a level of 5-6. The increased pH will have a mild antacid effect and will lead to an increase in beneficial probiotics (good bacteria). Increasing the pH of the stomach increases the pH of the body, which leads to healthy digestion and relief from the symptoms of indigestion. pH of subcutaneous fat Fat tissues of the body have an acidic pH, as excess acids are deposited in them. The body must store acid in fatty tissues when it cannot be excreted or neutralized by other means. That is why the acidic pH of the body is one of the factors of excess weight. Bone pH Bone has an alkaline pH because it is primarily composed of calcium. Their pH is constant, but if the blood needs pH adjustment, calcium is pulled from the bones.
Liver pH The liver has a slightly alkaline pH, the level of which is influenced by both food and drinks. Sugar and alcohol must be broken down in the liver, which leads to excess acid.
There are 6 alkaline minerals in nature that regulate the environment and can retain oxygen. These are Na, K, Mg, Ca, Fe, Mo (molybdenum), but it is very rare. The first four are of particular interest. Liquids consumed by a person must have a slightly alkaline reaction and be in the range from 7.5 to 8.5, which is physiological for the human body, since the specified pH allows one to better maintain the acid-base balance of body fluids. If the diet maintains an ideal balance of alkali-forming and acid-forming foods, then the resulting alkalis and acids neutralize each other and leave a pH - a neutral precipitate.
pH is the acid-base balance of aqueous media. Between meals, you can help your body normalize its pH balance by drinking water.
The best food is fresh vegetables, fruits, herbs, sprouted grains and legumes, but not thermally processed! The best health drink is pure water!
Poor nutrition is the cause of chronic acidification of the body
The diet of modern humans is characterized by an imbalance of hydrogen and bicarbonate ions, which causes lifelong, mildly pathogenic (pathogenic) existing systemic metabolic acidosis (acidification).
According to anthropologists, the diet of ancient man consisted of 1/3 lean meat and 2/3 plant foods. Under these conditions, the diet was exclusively alkaline.
The acid load of ancient man's food averaged minus 78.
The situation changed fundamentally with the emergence of agrarian civilization, when people began to eat a lot of grain crops, dairy products and fatty meat from domesticated animals.
But a particularly dramatic shift in nutrition occurred in the late 20th century, as industrially processed, acidic foods filled the diet.
These changes in the composition of the diet have been called risk factors in the pathogenesis of diseases of civilization: atherosclerosis, hypertension, osteoporosis, type 2 diabetes.
The acid load of modern human food is plus 48.
The modern diet is rich in saturated fats, simple sugars, table salt and low in fiber, magnesium and potassium. It is dominated by refined and processed foods, sugar, flour products, and many semi-finished products.
What is the food of modern man? These are pizza, chips, glazed cheese curds, newly-minted miracle dairy products, confectionery, and soft, sweet drinks. This food has acidic valencies. The body constantly strives to balance this ratio, maintaining a strictly defined pH level. This parameter has a significant impact on all biochemical processes in the body.
Norms
| Parameter | Norm |
| Color and transparency | The color of urine should be yellow with varying shades ranging from light to dark (but not brown). The color intensity varies depending on factors such as coloring foods (beets, blackberries, red berries), synthetic drugs, as well as vitamin complexes and contrast agents. Transparency must be complete. |
| Reaction (pH) | Depends on the nature of the diet. Normally, when eating meat foods, an acidic urine reaction will be observed; when eating a vegetarian or dairy diet, the urine reaction will be alkaline. |
| Protein | May be present in traces (up to 0.1 g/l). Sometimes protein in the urine of pregnant women can be a consequence of intense physical activity, anxiety and stress. |
| Glucose (sugar) | May appear in small quantities during the second half of pregnancy. Normally, these numbers are insignificant and do not cause concern. |
| Red blood cells | Must be missing. |
| Leukocytes | Normally, they should be within the following limits: general urine test - 6-8 in the field of view; urine analysis according to Nechiporenko - no more than 2000 per 1 ml. |
| Phosphates | Normally, they decrease sharply, since a significant part of phosphoric acid compounds goes to the formation of fetal skeletal bones, and after childbirth - to the formation of milk. |
Why is acidification of the body dangerous?
A decrease in pH in the body leads to a decrease in immunity and the appearance of more than 200 diseases, including farsightedness and cataracts, chondrosis, gallstones, kidney stones, and oncology. If one person experiences several diseases at the same time, there is a clear drop in blood pH. Naturally, restoring the pH to normal is a necessary condition for the treatment of these conditions.
When pH decreases, i.e. When acidity increases, it is noted:
- impaired immune response, viruses, bacteria, fungi multiply quickly. Back in 1932, Otto Warburg received the Nobel Prize in Chemistry for determining the living conditions of malignant tumors. Tumor cells (as well as bacteria and pathogenic microorganisms) proliferate when the blood is acidified, i.e. when the pH drops below 7.2-7.3 units. When the pH was normalized, the tumors first stopped growing and then resolved! If the blood pH is normal, foreign bacteria and microorganisms do not have conditions for reproduction.
- the skeleton is sacrificed, since for the purpose of alkalization, magnesium and calcium are washed out of the bones, which leads to the development of osteoporosis.
- In response to acidification, the body releases excess calcium into the bloodstream. The body strives to remove this excess, but, unfortunately, does not deposit it back into the bones, but on the surface of the bones and joints, as well as in the kidneys and gall bladder. Clouding of the lens begins, the development of cataracts accelerates, etc.
- Vitamins and microelements are poorly absorbed.
- diseases of blood vessels, heart, joints, and blood occur.
- Chronic acidification can also cause hypothyroidism, headaches, anxiety, insomnia, and swelling.
- Chronic weakness and muscle pain occur.
- the acidic reaction of saliva destroys teeth.
general description
As a rule, the indicators in any urine tests in pregnant and non-pregnant healthy women are practically the same. A general urine test is taken to determine any abnormalities during pregnancy and should accompany every visit to the doctor at all stages of pregnancy. Urinalysis is a mandatory step in prenatal diagnosis.
Color and transparency
The slightest cloudiness indicates a deviation from the norm and may indicate the presence of urolithiasis, salts, bacteria, leukocytes, and other formed elements.
Urine reaction (pH)
A decrease in the pH value of a pregnant woman’s urine indicates severe early toxicosis or food poisoning, which is accompanied by vomiting, diarrhea, i.e., loss of fluid from the body. A decrease in pH also occurs with a lack of potassium and magnesium in the body.
Protein in urine
More than 300 mg (proteinuria) per day of protein in the urine of a pregnant woman indicates kidney disease. During pregnancy, exacerbation of chronic kidney diseases may occur. If the protein is present in high titers, it means that the pregnant woman has developed one of the dangerous conditions: pyelonephritis or gestosis (late toxicosis). Proteinuria in pregnant women at 32 weeks indicates the development of nephropathy.
Glucose (sugar) in urine
If the level of sugar in the urine remains consistently high, then we are most likely talking about prediabetes or diabetes mellitus. To confirm (refute) the diagnosis, you need to donate blood for sugar and undergo a glucose tolerance test.
Ketone bodies in urine
Ketone bodies in urine during pregnancy indicate early toxicosis.
Red blood cells in urine
Their presence can be a sign of many diseases of the urinary system and gynecological pathology and requires a detailed in-depth examination.
Leukocytes in urine
A slight excess of the norm (leukocyturia) - up to 10-20 in the field of view, may be the result of low-grade inflammation or insufficiently thorough treatment (washing) of the external genitalia before taking tests. Leukocyturia with an increase in leukocytes to 40 or more in the field of view during pregnancy is a sign of pyelonephritis and other infectious kidney diseases.
Phosphates in urine
An increase in salts in the urine of a pregnant woman is a sign of disorders in the genitourinary system of the expectant mother.
Bacteriuria (presence of bacteria in urine)
Bacteriuria (the presence of bacteria in the urine) in pregnant women is evidence of cystitis or kidney disease. The appearance of bacteria together with an increase in leukocytes indicates a disease of an infectious nature. Most often this is a sign of acute pyelonephritis. Escherichia coli (Escherihia coli) is usually found in the urine of pregnant women. In order for treatment to give the expected effect, urine culture must be carried out for flora and sensitivity to antibiotics.