Ketone bodies
(syn.
acetone bodies
) - a group of organic compounds that are intermediate products of the metabolism of fats, carbohydrates and proteins. The appearance of increased amounts of ketone bodies in the blood and urine is an important diagnostic sign indicating a violation of carbohydrate and fat metabolism.
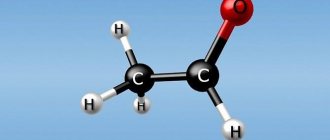
Ketone bodies include beta-hydroxybutyric acid (see Hydroxybutyric acids), acetoacetic acid (see) and acetone (see); they have a similar structure and are capable of mutual transformations:
What are ketone bodies?
The content of the article
These are compounds that arise as a result of biochemical changes in fats. Ketone bodies are formed in the liver, from where they enter the blood. In a healthy person, the rate of ketone bodies in the blood should not exceed 0.2 mmol/l.
Mechanism of formation of ketone bodies
Glucose is the main energy source for cells, but when it is deficient, energy comes from fats. There are 3 ketone bodies present in human urine and blood: B-hydroxybutyric acid, acetoacetic acid, and acetone.
Sources
- Masino, Susan. Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease. 2021.
- Dudchenko A.M. Energy metabolism and mechanisms of ATP stabilization. // Dissertation for the degree of Doctor of Medical Sciences. Research Institute of General Pathology and Pathophysiology of the Russian Academy of Medical Sciences. Moscow, 2003.
- National Task Force on the Prevention and Treatment of Obesity. National Institutes of Health. Very low-calorie diets // Journal of the American Medical Association. 1993. Vol. 270.
- Walter D. Longo, Mark P. Mattson. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metabolism. 2014 Feb 4; 19(2): 181–192.
- George A. Bray, William E. Heisel, Ashkan Afshin, Michael D. Jensen, William H. Dietz, et others. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocrine Reviews. 2021 Apr; 39(2): 79–132.
- Baranovsky, A. Yu. Dietetics: a guide. - St. Petersburg. : Publishing House "Peter", 2012., 1025 p.
- Owen, O. E., Morgan, A. P., Kemp, H. G., Sullivan, J. M., Herrera, M. G., and Cahill, G. F. Brain metabolism during fasting. // Journal of Clinical Investigations. 1967, 46, 1589–1595.
- Kristian H. Mikkelsen, Thomas Seifert, Niels H. Secher, Thomas Grøndal, Gerrit van Hall. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. Journal of Clinical Endocrinology and Metabolism. Feb 2015; 100(2):636-43.
- Mikhailova E.A., Lokoshko D.V., Bolshakova E.M. The use of controlled ketosis and exoketones as part of adjuvant therapy for various pathologies and as a means to improve quality of life. Eurasiascience. Collection of articles of the XXIX International Scientific and Practical Conference. 2021, pp. 35-40.
- Angela Poff, Andrew Koutnik, Sara Moss, Sahith Mandala. Exploring the Viability of Exogenous Ketones as Weight Loss Supplements. Current Developments in Nutrition. 2021, June, 3 (Suppl. 1).
- Joseph Mercola "The Cell on a Diet" Scientific discovery about the effect of fats on thinking, physical activity and metabolism. (Discoveries of the century: the latest research into the human body for the benefit of health). // Moscow: Publishing House “E”, 2021., 400 p.
- Murray AJ, Knight NS, Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. Federation of American Societies for Experimental Biology Journal. 2016; thirty.
- Volek, Jeff S., Daniel J. Freidenreich, Catherine Saenz. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2021., Vol. 65, no. 3., pp. 100 – 110.
- Adam Zajac, Stanisław Poprzecki, Adam Maszczyk, Miłosz Czuba, Małgorzata Michalczyk, Grzegorz Zydek. The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients. 2014, Jul.; 6 (7): pp. 2493 – 2508.
- McDonald, Tanya JW, Mackenzie C. Cervenka. The Expanding Role of Ketogenic Diets in Adult Neurological Disorders // Brain Sciences. 2021., Vol. 8 (August)., P. E148.
- Stafstrom, C. E., J. M. Rho. The ketogenic diet as a treatment paradigm for diverse neurological disorders // Frontiers in Pharmacology. 2012., Vol. 3.
- Vanitallie, T.B., Nonas, C., Di Rocco, A., Boyar, K., Hyams, K., and Heymsfield, S.B. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. // Neurology, 2005, 64, 728–730.
- Newport MT, VanItallie TB, Kashiwaya Y., King MT, Veech RL A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer's disease. Alzheimers & Dementia. 2015; 11:99–103.
- Peter A, McPherson C., McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. Journal of Physiology and Biochemistry. Mar 2012; 68(1):141-51.
- Brianna J. Stubbs, Rhys D. Evans, Peter Santer, Jack J. Miller, Olivia K. Faull, Snapper Magor-Elliott et others. On the Metabolism of Exogenous Ketones in Humans. Frontiers in Physiology. 2017; 8:848.
- Interdepartmental strategy for the formation of a healthy lifestyle, prevention and control of non-communicable diseases until 2025: https://www.gnicpm.ru/UserFiles/PROEKT_STRATEGII_NIZ-210616.pdf. [Electronic document] Website of the Federal State Budgetary Institution "National Medical Research Center for Preventive Medicine" of the Ministry of Health of the Russian Federation.
Ketones in urine - pay attention
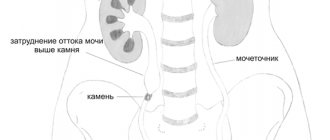
The condition in which ketone bodies are present in the urine is called ketonuria. If ketone bodies are found in the urine, this means that the body is using fats for energy. There can be many reasons that cause ketonuria, along with associated symptoms.
Symptoms occur when the body is unable to utilize carbohydrates. which leads to fat burning in diabetic patients. In patients without diabetes, ketonuria may be caused by a lack of carbohydrates. The main thing is to determine the cause of ketones in the urine as soon as possible and begin appropriate treatment. Every day of delay matters.
Symptoms that may accompany ketonuria
If there are ketones in the urine or symptoms that may be associated with the presence of ketones in the urine, you should immediately consult an endocrinologist. Symptoms appear when ketonuria is already progressing.
Signs that may indicate the presence of ketone bodies:
- characteristic - fruity smell from the mouth - acetone, which is present in the exhaled air, is responsible for it ( this is the most characteristic symptom of ketonuria);
- high blood glucose levels greater than 300 mg/dL;
- skin color has changed, redness or vice versa – pale color;
- gastrointestinal symptoms – diarrhea, vomiting, abdominal pain;
- frequent infections;
- chronic fatigue;
- dry mouth and increased thirst;
- polyuria.
Study of urinary ketone bodies
Ketone bodies can be detected using urine strip testing. You need to donate mid-stream morning urine, when the first portion of urine goes into the toilet, the middle part goes into a specially prepared sterile container, and the last part goes back into the toilet.
Urine analysis testing
Before collecting urine, you need to wash yourself. During the period leading up to the urinalysis, do not change your diet or do too much exercise.
A normal urine body test for ketones is negative. If ketone bodies are detected in the urine, treatment should be started immediately.
Causes of ketone bodies in urine
- Diabetes, most often type 1 diabetes
. A diabetic's body cannot cope with burning carbohydrates. The reason for this is a deficiency of insulin, due to which fat reserves are mobilized for energy. As a result of these changes, ketone bodies are formed. Excess ketones accumulate in the blood, a condition called ketonemia. The body suffering from diabetes, wanting to get rid of excess ketones, releases them along with urine, as well as with exhaled air. Most often, ketone bodies in the urine are accompanied by hyperglycemia, that is, high levels of glucose in the blood, as well as the presence of glucose in the urine (glucosuria). An excess of ketone bodies in the body leads to the development of ketoacidosis. This is a life-threatening condition. Factors predisposing to ketoacidosis include: improper use of insulin, sudden cessation of insulin therapy, chronic infections, life-threatening conditions: stroke, heart attack, pancreatitis. - Low carbohydrate diet
. During prolonged fasting, ketone bodies are formed during excessive B-oxidation of fats. - Chronic alcoholism.
- Other reasons
- temporarily in case of severe vomiting, chronic fever, excessive physical exertion.
Having determined the cause of the presence of ketone bodies in the urine, the doctor will begin appropriate treatment.
Hyperketonemia syndrome in children and adolescents: pathogenesis, causes, diagnosis
Part 1
Ketone bodies (acetone bodies) are a group of organic compounds that are intermediate products of the metabolism of carbohydrates, fats, and proteins. Keto acids are a product of the metabolism of acetyl-coenzyme A (acetyl-CoA), which, in the event of food deficiency, is formed either from its own proteins or from fat. Ketone bodies include β-hydroxybutyric acid (β-hydroxybutyrate), acetoacetic acid (acetoacetate), and acetone.
Physiology of energy metabolism
Ketone bodies are synthesized mainly in the mitochondria of liver cells from acetyl-CoA, which combines several key metabolic processes of the cell. The main function of acetyl-CoA is to deliver carbon atoms with an acetyl group to the tricarboxylic acid cycle (TCA cycle, Krebs cycle) so that they are oxidized to release energy. The content of acetyl-CoA determines the direction of cellular metabolism at a given moment: whether the synthesis and accumulation of glycogen, fat and protein synthesis will occur or, conversely, previously accumulated energy reserves in the body will be consumed.
The formation of ketone bodies is a physiological process and a necessary part of energy metabolism. During this exchange, “combustion” and intertransformation of carbohydrates, proteins, fats and other energy substrates occurs with the formation of energy, which is either converted into heat or accumulated in the form of adenosine triphosphate (ATP).
In conditions of energy deficiency in the body, the restoration of energy reserves is possible due to the activation of gluconeogenesis or the synthesis of ketone bodies (ketogenesis).
Ketone bodies play an important role in maintaining the body's energy balance, and activation of ketogenesis is more appropriate and optimal for the body under these conditions. Ketone bodies not only preserve the structural proteins of the body, inhibiting the secretion and action of glucagon, a powerful stimulator of gluconeogenesis, but also regulate and limit the intensity of ketogenesis through a feedback mechanism [1].
Gluconeogenesis is a metabolic pathway for the biosynthesis of glucose from non-carbohydrate precursors, active in the liver, kidneys, and small intestine. When glycogen reserves in the body are depleted, the liver switches to its synthesis through gluconeogenesis. The substrates of gluconeogenesis are: pyruvic acid (pyruvate), lactic acid (lactate), glycerol, glucogenic amino acids, fatty acids.
Transformation of pyruvic acid is possible in two ways - aerobic or anaerobic. Glycolysis, or the Embdem–Meyerhoff pathway, is the main pathway for glucose utilization in cells. One molecule of glucose is converted into two molecules of pyruvic acid. The conversion of pyruvate to acetyl-CoA occurs with the participation of a set of enzymes structurally united in the pyruvate dehydrogenase complex (PDC).
Under aerobic conditions, pyruvate enters the mitochondria. The formed acetyl-CoA is oxidized to CO2 and H2O in the Krebs cycle. The main part of glucose is spent on ATP synthesis in the process of oxidative phosphorylation.
If the oxygen content is insufficient, as can be the case in skeletal muscles during intense physical activity or in tissues where there are no or very few mitochondria (red blood cells, white muscles, retinal cells, adrenal medulla), glycolysis is the final energy process, resulting in of which pyruvate is converted into lactate, and the latter into a product of anaerobic glycolysis. It is formed under any conditions of the body in red blood cells and working muscles. Excess lactate enters the liver, where it is synthesized back into glucose (gluconeogenesis). Thus, lactate is constantly used in gluconeogenesis.
During muscular work, glucose in the myocyte is used not only for energy needs, but also to ensure a constant flow of oxaloacetate into the TCA cycle. Moreover, with increasing duration of exercise, the “energetic” role of glucose decreases [2].
Acetyl-CoA is a key metabolite of lipid metabolism. It is formed during β-oxidation of fatty acids in liver mitochondria. In the mitochondrial matrix of liver cells, fatty acids are oxidized in the Knopp–Linen cycle. A key player in this process is L-carnitine, which transports long-chain fatty acids into mitochondria through the inner membrane of the latter. This process is insulin dependent. Normally, citrate is formed by the condensation of oxaloacetate and acetyl-CoA with the participation of the enzyme citrate synthetase.
Amino acids (leucine, tyrosine, phenylalanine), formed as a result of the breakdown of muscle proteins, are included in gluconeogenesis during prolonged fasting or prolonged muscle work. During catabolism, they are converted to acetoacetate and can be used in the synthesis of ketone bodies.
The inclusion of lactate, glycerol and amino acids in gluconeogenesis depends on the physiological state of the body. The oxidation of one molecule of β-hydroxybutyrate produces CO2 and H2O and ensures the synthesis of 27 ATP molecules.
Biological role of ketone bodies
Ketone bodies play an important role in maintaining energy balance. The resulting acetone bodies enter the blood from hepatocytes and are carried to the cells of various organs. Acetone bodies are normally utilized quite well by cells of peripheral tissues, especially in skeletal muscles and myocardium, which receive a significant portion of the energy they need through the oxidation of acetone bodies. The main pathway for activation of acetoacetate in cells is the pathway involving thiaphorase. Hepatocytes do not have this enzyme. That is why acetoacetate formed in hepatocytes is not activated or oxidized, thereby creating conditions for the “export” of acetoacetate from hepatocytes into the blood.
In healthy people, with increased lipolysis, the rate of utilization of ketone bodies, which are important sources of energy during muscular work and fasting, increases. Skeletal muscles and kidneys use ketone bodies even at low concentrations in the blood. Only the cells of the central nervous system practically do not utilize acetone bodies under normal conditions [3].
Ketone bodies are part of a regular metabolic mechanism to prevent excessive mobilization of fatty acids and inhibit proteolysis, which preserves the body's structural proteins. Normally, ketone bodies stimulate the release of insulin from the pancreas, which inhibits lipolysis and thus limits the delivery of lipids to the liver and, accordingly, ketogenesis. During fasting, ketone bodies are one of the main sources of energy for the brain [4]. Normally, the processes of synthesis and use of ketone bodies are balanced. The concentration of ketone bodies in the blood and tissues is usually very low, so the content of ketone acids in the blood plasma is normally present in extremely low quantities and amounts to 0.1–0.3 mmol (0.03–0.2 mmol/l for acetone) .
The presence of ketone bodies in urine always indicates the development of a pathological condition in the body. Ketone bodies are removed in the urine in varying quantities: acetone - 3-4%, acetoacetic acid - 30-40%, β-hydroxybutyric acid - 60-70%.
Laboratory tests that detect ketonuria are based on reactions with acetoacetate and acetone, since they do not react with β-hydroxybutyrate. To qualitatively determine the content of ketone bodies in urine, Lange, Legal, Lestrade, and Gerhard color tests are used. The amount of acetoacetate in a urine test is measured in pluses (from one + to four ++++). The presence of +++ corresponds to an increase in the level of ketone bodies by 400 times, ++++ - by 600 times. Physiological ketosis can be detected during fasting, heavy muscular work, and in newborns [5].
Causes of Excess Ketosis
Hyperketonemia that occurs under pathological conditions is associated with the dissociation of ketogenesis in the liver and the utilization of ketone bodies in other organs, that is, either the rate of synthesis of ketone bodies in the liver exceeds the rate of their utilization by peripheral tissues of the body, or their utilization as a source of energy in other organs is impaired.
An increase in the content of ketone bodies in the body is primarily caused by a deficiency of carbohydrates to provide the body with energy, an overload of proteins and fats against the background of a lack of easily digestible carbohydrates in the diet, exhaustion of the body, obesity, disruption of endocrine regulation (diabetes mellitus, thyrotoxicosis, etc.), poisoning, skull injuries etc. [4].
Intensive formation of ketone bodies also occurs in the presence of oxaloacetate deficiency, since the latter is the main regulator of the TCA cycle. There is usually a balance in the cell between the production of acetyl-CoA (from glucose, fatty acids or amino acids) and the amount of oxaloacetate. The source of oxaloacetate is glucose (synthesis from pyruvate), intake from fruit acids of the cycle itself (malic, citric), formation from aspartic acid. With an insufficient amount of oxaloacetate in the blood plasma, as observed in pathological conditions, the concentration of ketone bodies can increase significantly. Without having time to oxidize and being quite strong organic acids, they cause the development of metabolic ketoacidosis.
Stimulation of ketogenesis during food deficiency, stress, and prolonged vomiting is a compensatory process during which the energy deficit is replenished by keto acids.
Hyperketonemia with a shift in pH to the acidic side can be observed when the Krebs cycle is inhibited, in which the “burning” of ketone bodies occurs.
The rate of formation of ketone bodies also depends on the rate of oxidation of fatty acids in the liver, and the oxidation process accelerates with increased lipolysis (fat breakdown) in adipose tissue. Intensive formation of keto acids also occurs when so-called ketogenic amino acids (leucine, tyrosine, phenylalanine, isoleucine), some proteins and large amounts of fats are taken with food [5].
In moderate ketosis, mainly acetoacetate and β-hydroxybutyrate circulate in the blood. Acetone is formed only at high concentrations of ketone bodies and is primarily eliminated through the lungs.
However, under extreme conditions, glucose can be synthesized from ketone bodies using gluconeogenesis, which serves as a source of energy for the functioning of the central nervous system.
Acetone bodies, accumulating in the blood and tissues, have an inhibitory effect on lipolysis, especially with regard to the breakdown of triglycerides in adipocytes. A decrease in the level of lipolysis in adipose tissue cells leads to a decrease in the influx of higher fatty acids into hepatocytes and to a decrease in the rate of formation of acetone bodies and, consequently, a decrease in their content in the blood.
With energy deficiency as a result of changes in hormonal status and the action of intracellular regulatory mechanisms, the rate of fat mobilization increases and gluconeogenesis from amino acids and glycerol increases. Lipolysis is activated by glucagon and adrenaline. Long-term stimulation of ketogenesis or disruption of ketolysis processes lead to a change in the buffer capacity of the blood, and when blood ketone bodies are present in excessively high concentrations, life-threatening decompensated ketoacidosis occurs.
This picture is typical for severe type 1 diabetes mellitus, hypoglycemia, prolonged fasting, stress of various etiologies, liver diseases, heavy and prolonged muscular work [6].
Ketone bodies are water-soluble acids, therefore, unlike fatty acids, they can pass through the blood-brain barrier and serve, along with glucose, as a source of energy for nervous tissue, especially after 3-5 days of fasting, when the concentration of ketone bodies in the blood increases significantly.
Diabetes
Type 1 diabetes mellitus is the most common cause of ketosis and ketoacidosis. The leading role in the pathogenesis of ketoacidosis is played by absolute insulin deficiency, leading to a decrease in glucose utilization by insulin-dependent tissues and, accordingly, hyperglycemia and severe energy starvation in them. Insulin affects all types of metabolism. Insulin deficiency in diabetes mellitus causes a sharp increase in the blood level of all counter-insular hormones (glucagon, cortisol, catecholamines, thyroxine, etc.). They stimulate the mobilization of lipids from fat depots and the delivery of fatty acids to organs, which is an adaptive mechanism that supplies an alternative oxidation substrate in conditions of decreased glucose utilization by cells. The activity of lipoprotein lipase (LP lipase) of adipocytes decreases, so free fatty acids do not enter the adipose tissue. The effect of glucagon begins to predominate, stimulating ketogenesis in the liver and hormone-sensitive triacylglycerol lipase (TAG lipase) in adipocytes.
In diabetes mellitus, the β-oxidation product of fatty acids, acetyl-CoA, begins to form in excess quantities. However, the ability of the Krebs cycle to utilize this product is significantly reduced, since β-oxidation of free fatty acids in mitochondria depends on their transport across the mitochondrial membrane. And this process is insulin dependent.
If it is hindered, then fatty acids are quickly converted into acyl-CoA, from which acetyl-CoA is formed. The Krebs cycle and fatty acid resynthesis are unable to fully utilize the excess acetyl-CoA produced, especially since the citrate cycle is inhibited by this excess. Normally, citrate is formed by the condensation of oxaloacetate and acetyl-CoA with the participation of the enzyme citrate synthetase. The activity of the latter during decompensation of diabetes is reduced, in particular, due to the inhibitory effect of ATP, which is formed in excess during the oxidation of fatty acids.
The formation of oxaloacetate is also reduced, since the NADH/NAD+ ratio increases as a result of increased oxidation of free fatty acids and increased gluconeogenesis. This leads to insufficient citrate formation and accumulation of acetyl-CoA.
This process is facilitated by an increase in the content of carnitine in the liver (especially under conditions of activation of the effects of glucagon). Carnitine stimulates the transport of fatty acids into the mitochondria of liver cells, where they undergo β-oxidation, significantly accelerating ketogenesis.
As a result, excess acetyl-CoA becomes a source of formation of large quantities of ketone bodies: β-hydroxybutyric acid, acetoacetic acid and acetone.
In patients with type 1 diabetes mellitus, protein metabolism is disrupted, which is characterized by the predominance of catabolic processes as a result of activation of the process of gluconeogenesis from glucogenic amino acids and a decrease in the permeability of cell membranes to amino acids, which leads to a lack of free amino acids in the tissues and disruption of the protein synthesis process [6].
Tissue hypoxia causes activation of anaerobic glycolysis and an increase in lactate content, which cannot be utilized as a result of lactate dehydrogenase deficiency against the background of insulin deficiency. This aggravates the imbalance of the body's acid-base balance and leads to lactic acidosis.
An active increase in the content of ketone bodies during decompensation of diabetes mellitus is associated not only with increased production, but with reduced peripheral utilization. With excessive accumulation of ketone bodies, the buffering capacity of the blood is quickly depleted, which leads to the development of decompensated metabolic ketoacidosis. Ketone bodies begin to be excreted in the urine in the form of sodium salts, and acetone is also excreted in the exhaled air.
The consequence of increasing the concentration of acetoacetate is the acceleration of the formation of acetone, which has a toxic property. It dissolves in the lipid components of cell membranes and disorganizes them. All tissues of the body suffer, and most of all the cells of the nervous tissue. The functioning of many enzymatic systems is disrupted. This may manifest itself as loss of consciousness [7].
In type 2 diabetes mellitus, minimal insulin production remains, which explains the rarity of the development of lipolysis and the state of ketoacidosis and ketoacidotic coma with increasing hyperglycemia.
Hypoglycemia and hypoglycemic conditions
Ketotic hypoglycemia is the most common cause of low blood glucose. Stimulation of ketogenesis under conditions of hypoglycemia syndrome is associated with activation of lipolysis processes during severe energy starvation. As glycogen reserves in the liver are depleted, the content of glucagon, adrenaline, norepinephrine, cortisol, and growth hormone increases, which stimulate gluconeogenesis [8, 9].
Fatty acids are extensively metabolized from adipose tissue to provide a source of energy for muscle activity and available glucose for the central nervous system. Fatty acids are oxidized in the liver to form ketone bodies - acetoacetate and β-hydroxybutyrate.
Hypoglycemia due to enzyme deficiency
Disorders of glycogen metabolism associated with its pathological deposition are manifested by glycogen diseases. This is a group of hereditary disorders that are based on a decrease or absence of activity of enzymes that catalyze reactions of synthesis (aglycogenosis) or breakdown of glycogen (glycogenosis).
Defect of the enzyme glucose-6-phosphatase (Gierke's disease). The primary disorder in Gierke's disease (type 1 glycogenosis) occurs at the genetic level. It consists of a complete or almost complete inability of cells to produce glucose-6-phosphatase, which ensures the cleavage of free glucose from glucose-6-phosphate. As a result, glycogenolysis is interrupted at the level of glucose-6-phosphate and does not go further. Dephosphorylation with the participation of glucose-6-phosphatase is a key reaction not only of glycogenolysis, but also of gluconeogenesis, which, therefore, in Gierke's disease is also interrupted at the level of glucose-6-phosphate.
The occurrence of sustained hypoglycemia, which in real conditions is inevitable due to the lack of glucose entering the blood as the final product of glycogenolysis and gluconeogenesis, in turn leads to a constant increased secretion of glucagon as a stimulator of glycogenolysis. Glucagon, however, when this process is interrupted, can only continuously stimulate its initial stages without benefit to the body.
Mental and somatic development, as well as biochemical status (increased levels of triglycerides, cholesterol, hyperuricemia, hypophosphatemia) are severely impaired in these patients. The glucose content in fasting plasma is constantly reduced, and therefore, even with short-term fasting, hypoglycemic cramps, ketonuria and metabolic acidosis develop. The latter is caused not only by hyperketonemia, but also by increased accumulation and formation of pyruvate and lactate in the blood, which is the result of impaired gluconeogenesis. Characteristic features of the disease are: mental retardation, growth retardation, obesity, osteoporosis, large belly (a consequence of enlarged liver and kidneys), xanthomatosis, retinal lipemia, hemorrhagic diathesis.
The diagnosis is based on the clinical picture, decreased glucose levels and increased concentrations of lipids and lactate in the blood. Plasma glucose levels remain virtually unchanged after glucagon administration. However, the lactate content in the blood increases after its administration. Liver biopsy and special histochemical methods confirm the deficiency of the corresponding enzymes [10].
Some hypothalamic-pituitary syndromes may be accompanied by hypoglycemia: Lawrence-Moon-Biedl-Bordet syndrome, Debreu-Marie syndrome, Pechkranz-Babinsky syndrome (adiposogenital dystrophy).
Lawrence–Moon–Biedl–Bordet syndrome is characterized by obesity, hypogonadism, mental retardation, retinal degeneration, polydactyly, and profound degenerative changes in the hypothalamic-pituitary system.
Debre-Marie syndrome is a disease caused by hyperfunction of the posterior lobe of the pituitary gland and hypofunction of the adenohypophysis. Appears in early childhood. Patients are infantile, short, and overweight. The clinical picture is characterized by a violation of water metabolism with oliguria and oligodypsia, and the urine density is high. Mental development is not impaired.
Pechkranz-Babinski syndrome - the cause of the disease is considered to be organic and inflammatory changes in the hypothalamus, which lead to obesity, abnormalities of skeletal development and hypoplasia of the genital organs.
Obesity
The rapid increase in obesity in all age groups of people is largely associated with changes in lifestyle: a decrease in physical activity (computerization, motorization, urbanization), changes in the nature of nutrition (overeating), etc. Excessive energy intake or a decrease in its expenditure leads to weight gain body and the development of obesity.
Adipose tissue has high metabolic activity. It continuously undergoes intensive metabolic processes, such as the synthesis and hydrolysis of lipids, the synthesis of fatty acids, including from carbohydrates, their esterification into triglycerides or neutral fat, their deposition and breakdown to form fatty acids, and the use of the latter for energy purposes.
Obesity leads to significant metabolic and metabolic disorders. They are characterized by hyperinsulinism and impaired glucose tolerance; insulin resistance caused by disruption of insulin receptor relationships; an increase in the content of free fatty acids in the blood, a tendency to ketogenesis during fasting and hypertriglyceridemia. With obesity, the activity of lipolytic enzymes in adipose tissue decreases: triglyceride lipase, which leads to their accumulation, and lipoprotein lipase. The breakdown of lipoproteins is reduced. Hypertrophied adipocytes respond weaker than hyperplastic ones to adrenaline, norepinephrine and other lipolytic substances [11].
An important manifestation of interstitial metabolic disorders in obesity is ketosis, associated with increased lipolysis and excess supply of free fatty acids to the liver. The reaction rate in the TCA cycle is reduced because oxaloacetate is used for gluconeogenesis. As a result, the rate of formation of acetyl-CoA exceeds the ability of the TCA cycle to oxidize it. Acetyl-CoA is used for the synthesis of ketone bodies. Due to an excess of ketone bodies, their utilization is impaired.
This is facilitated by the observed hypoglycemia, hyperlipidemia, and ketonemia when using a long-term hypocaloric diet. And the concomitant carbohydrate deficiency inhibits the use of acetyl-CoA in the Krebs cycle. When there is a deficiency of carbohydrates in the body, there is a lack of energy in the cells. Lipolysis increases [12].
An excess of non-esterified fatty acids entering the liver causes the development of fatty infiltration in it, which disrupts the oxidation and removal of lipoproteins from the liver, causing the accumulation of ketone bodies. To effectively use the products of fat breakdown, products of carbohydrate metabolism are needed - fats “burn” in the flame of carbohydrates.
Read the end of the article in the next issue.
V. V. Smirnov1, Doctor of Medical Sciences, Professor A. V. Simakov
Federal State Budgetary Educational Institution of Russian National Research University named after. N. I. Pirogova Ministry of Health of the Russian Federation, Moscow
1 Contact information
Ketones in urine - rate of acetone in urine and interpretation of the result
The norm for ketones in urine is the absence of ketone bodies. Any positive result (positive ket in the urine) means a problem in the body and requires further diagnosis. Traces of ketones in urine or low levels, such as urinary ketones 5 or urinary ketones 15 mg/dL, may indicate:
- intense physical effort;
- lack of calories in the diet;
- vomiting;
- fever;
- burns;
- alcohol consumption.
Intense physical effort
Fever
Most often, mild ketonuria occurs without symptoms that may indicate diabetes or ketoacidosis.
Excessively high levels of urinary ketones, that is, a test result indicating a urine ketone level of 50 mg/dL or more, may indicate more serious health problems such as severe debilitating diseases, thyroid disease, or renal glycosuria. High ketones in urine also accompany decompensated or undiagnosed and untreated diabetes.
Increasing values
- Decompensated diabetes mellitus (the higher the level of ketones, the higher the risk of developing hyperglycemic coma);
- Poorly designed diet: low-carbohydrate or high-protein diets, fasting, fasting;
- Cachexia (depletion of the body) in severe form;
- Dehydration;
- Inflammatory processes in the intestines, leading to impaired absorption of food;
- Oncological diseases: cancer of the thyroid gland, adrenal glands, liver, intestines, gastric mucosa, brain, as well as leukemia, leukemia;
- Anemia (anemia), causing massive death of red blood cells;
- Liver diseases: cirrhosis, hepatitis, liver failure, etc.;
- Acute alcohol intoxication, which leads to damage to the liver parenchyma;
- Poisoning with heavy metal salts or atropine;
- Serious injuries (including concussion), massive burns;
- Diseases of the thyroid gland, for example, thyrotoxicosis (hypersecretion of iodinated hormones);
- Toxicosis during pregnancy;
- Viral, bacterial or infectious processes accompanied by high fever;
- Insufficient production of blood cells: thrombocyto-, lymphocyto- and granulocytopenia;
- Narrowing of the pylorus of the stomach.
In healthy people, ketones may also temporarily increase if:
- significant protein consumption for several days in a row;
- insufficient fluid intake;
- intense physical activity, heavy lifting, sports activities;
- general hypothermia of the body.
Ketones in a child's urine - reasons
Acetone in the urine in children is most often accompanied by vomiting or fever. Sometimes this is a sign of malnutrition or adrenal insufficiency - then high levels of ketone bodies in children's urine are accompanied by hypoglycemia, that is, low blood glucose levels. Conversely, ketones in the urine when glucose levels are low and the liver is enlarged may indicate the presence of glycogenosis.
Ketone bodies and glucose in a child's urine may indicate diabetes; Type 1 diabetes is most common in young adults. To determine whether a child has ketones, it is necessary to first measure fasting blood glucose levels. Further diagnosis depends on the glucose result.
What else is there to benefit from taking T8 ERA Exogenous Ketones?
We have already talked in more detail about the therapeutic, preventive and stimulating effects of ketone bodies on various tissues and organs of our body in a previously published article. Here we just list the main points:
- Increased physical and psychological endurance [12].
- Reduced fatigue and improved exercise tolerance [13].
- Stimulation of growth and reproduction of muscle cells [14].
- Protection of nervous tissue from damage during intoxication [15].
- Protection of the brain during oxygen starvation caused by a sedentary lifestyle, cervical osteochondrosis and other reasons typical for residents of large cities [16].
- Prevention of Alzheimer's and Parkinson's diseases, as well as senile dementia [17, 18].
- Psychotonic and antidepressant effects [19].
- Improving a person’s cognitive capabilities, which allows him to maintain concentration and performance longer [20].
Ketone bodies in urine during pregnancy - what do they show?
Ketones in urine are not uncommon during pregnancy. Women who suffer from frequent vomiting in the first trimester of pregnancy show the presence of ketones very often. In addition, lack of appetite during pregnancy or skipping an evening meal the day before urine tests can lead to an increase in ketone bodies in the blood and urine.
Since high levels of acetone in the urine of a pregnant woman may indicate gestational diabetes, the condition always requires further diagnosis. Then, in addition to ketones, glucose may also be observed in the urine of a pregnant woman, and there may also be symptoms of ketoacidosis.
And what's wrong with that?
This evolutionary mechanism (glucose = pleasure), as they say, is “hardwired into our subcortex.” And throughout almost the entire history of mankind, it worked perfectly. But at some point, the development of civilization led to the fact that sweets from rare delicacies turned into everyday food, access to which is unlimited. Only this mechanism has not gone away. And it is precisely this that is the reason that it is so difficult for us to consciously control the consumption of jam, pastries, cakes, sweets, ice cream, pastries, and other foods rich in simple carbohydrates. Moreover, if adults are still able to somehow cope with such temptations, then for children this task is not at all possible - they are simply not able to resist what evolution has laid in us [4].
Traces of ketones in urine - treatment
Ketonuria is not a disease, but a symptom of a disease, so it cannot be cured. To restore urinary ketone levels to normal, the underlying condition must be eliminated or treated. It is necessary to compensate for disturbances in acid-base and water-electrolyte metabolism. This is especially important in the case of diabetes, a complication of which can be ketone coma.
To get rid of ketones in the urine in diabetes, it is necessary to normalize the levels of glucose and insulin in the blood. In case of ketonuria caused by malnutrition, it is necessary to change the diet to a high-calorie diet and ensure an adequate caloric balance. During vomiting, antiemetic drugs are prescribed.
ONLINE REGISTRATION at the DIANA clinic
You can sign up by calling the toll-free phone number 8-800-707-15-60 or filling out the contact form. In this case, we will contact you ourselves.
If you find an error, please select a piece of text and press Ctrl+Enter