What is pyelectasia
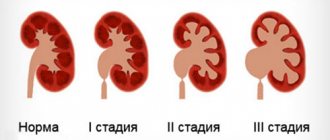
Pyeelectasia is an enlargement of the renal pelvis . Pyeelectasis is 3-5 times more common in boys than in girls. Both unilateral and bilateral pyeloectasia occur. Mild forms of pyelectasis often go away on their own, while severe forms sometimes require surgical treatment.
Cause of renal pyelectasis in the fetus
If there is an obstacle in the way of the natural outflow of urine, urine will accumulate above this obstacle, which will lead to expansion of the renal pelvis. Pyeelectasis in the fetus is diagnosed by routine ultrasound examination at 18-22 weeks of pregnancy.
Is pyeelectasis dangerous?
Moderate expansion of the renal pelvis, as a rule, does not affect the health of the unborn child. In most cases, during pregnancy, spontaneous disappearance of moderate pyelectasis is observed. Severe pyelectasis (more than 10 mm) indicates a significant difficulty in the outflow of urine from the kidney. Difficulty in the outflow of urine from the kidney may increase, causing compression, atrophy of the kidney tissue and decreased kidney function. In addition, a violation of the outflow of urine is often accompanied by the addition of pyelonephritis, an inflammation of the kidney that worsens its condition.
Slightly more often, dilation of the renal pelvis is detected in fetuses with Down syndrome. However, this marker refers to the “minor” markers of Down syndrome, therefore, detecting only dilation of the renal pelvis does not increase the risk of having Down syndrome and is not an indication for other diagnostic procedures. The only thing you need to do before giving birth is to undergo a control ultrasound at 32 weeks and once again assess the size of the renal pelvis.
Megaureter in a child, causes, symptoms and treatment in children.
Histological findings: Surgical pathology is divided into 5 histological varieties (types): (1) abnormalities of circularly oriented muscle fibers with hypertrophy and hyperplasia, (2) fibrosis of the ureteral wall with a deficiency of normal muscle fibers and thinning of the ureter, (3) hypoplasia and atrophy of all muscular structures of the ureter, (4) absence of oblique muscles, (5) normal anatomy of the ureter.
TREATMENT
Drug therapy: Typically, doctors can monitor symptoms, perform periodic radiological examinations, and prescribe antibacterial prophylaxis for exacerbations of pyelonephritis. In the case of a decrease in the size of the ureter and stable kidney function, parenchyma continues to grow and prognostically long-term results in such patients (follow-up for more than 8 years) are excellent
- Antibiotics: Amoxicillin (Amoxicillin)
Penicillin
Surgical treatment: Megaureter detected in a newborn or infant may require drainage due to exacerbations of pyelonephritis, since antibacterial therapy may have only a short-term effect. Additionally, a huge dilatation of the ureter can be drained with a ureterostomy, pyelostomy or nephrostomy, which very often make it possible to reduce the size of the ureter and improve its contractility; such patients are less likely to require narrowing of the ureter during implantation.
Radical treatment of the defect in newborns and children in the first months of life is rarely performed. It is advisable, if the condition allows, to postpone the operation until 3-5 months. age when the child grows and gains weight up to 70-100%. At this age, renal function and urodynamic status can be more accurately assessed. Children undergo maturation processes and often there is no need for surgery. At an older age, it is possible to use endoscopic methods for correcting the defect as a method of preparation for the main operation.
The main goal of surgical treatment is to eliminate obstruction, restore normal urine flow and the function of the collector system, perform ureterocystone implantation (implantation of the ureter into the bladder) with anti-reflux protection, and preserve kidney function without long-term complications. These goals correspond to 2 main surgical approaches:
- Mobilization of the distal ureter, resection of the obstructive segment and reimplantation (with or without constriction) of the dilated ureter (to provide a better anti-reflux mechanism)
- Extended narrowing of the ureter from the renal pelvis in a distal direction theoretically helps to increase peristalsis in the smaller diameter ureter. In practice this is rarely necessary.
In experienced hands, following these two principles can achieve excellent long-term results. Significant differences are not obvious statistically or clinically.
Preoperative details: The operation is performed if there are no signs of urinary infection. Blood loss is not significant and usually does not require transfusion.
Intraoperative details: The choice of incision depends on access to the upper segment of the ureter, the lower segment, or to expose the entire ureter. Intravesical and extravesical isolation of the ureter are used, alone or in combination, to isolate the ureter throughout.
The blood supply to the ureter is very carefully preserved. The trunks coming from the aorta, renal artery, gonadal artery, internal iliac artery should not be damaged during prolonged narrowing of the ureter. Impaired blood supply to the ureter can lead to the formation of extended stenoses with subsequent progressive loss of kidney function.
The narrowing can be extensive and extended, including removal of ureteral kinks, however, a reduction in ureteral diameter of more than 10 Fr is recommended to be avoided due to the risk of stenosis.
The length of the neocystostomy (anti-reflux) tunnel is 4-6:1 to the diameter of the ureter to prevent postoperative reflux. Postoperative ureteral drainage is used at the discretion of the surgeon.
Postoperative details: Patients continue antibacterial prophylaxis for several months after surgery without interruption, under the control of urine and general clinical status.
Follow-up: Ultrasound is repeated, assessment of the size of the pelvis and ureter, gradual disappearance of signs of hydronephrosis and hydroureter after surgery. As long as there are doubts, close monitoring of the patient continues, including cystography and excretory urography. Only if the operation is clearly successful can the patient be limited to ultrasound as a dynamic observation.
COMPLICATIONS
Surgical complications are uncommon and often associated with obstruction.
- Reflux on the side of surgery occurs in 2% of patients.
- Reflux on the opposite side is detected in 10% of patients within 1 year after surgery.
- Ureteral stenosis (1%): Early balloon dilatation or ostial dissection is highly effective. Extended stenosis of up to 1.5 cm or more occurs when the blood supply to the segment is disrupted and requires reoperation.
- Incomplete emptying of the bladder requires catheterization (5%) and a course of electrical stimulation. Permanent persistence of this symptom is rare.
LONG-TERM RESULTS AND PROGNOSIS
According to research, the long-term results of such surgical tactics are 98% successful in large groups of observations. Failures were usually associated with the occurrence of reflux and were often associated with an unconstricted replantant (ureter). Reoperation with ligation and repeat neocystostomy is usually performed.
Children who previously suffered from frequent exacerbations of secondary pyelonephritis may have episodes of exacerbation of the inflammatory process. Such patients are prescribed prolonged antibacterial prophylaxis, which limits the pathological effect of infection on kidney growth. Additionally, we emphasize that urinary tract infections are controlled from the period of pregnancy.
Kidney function is the key to determining the prognosis of the disease. Renal tissue dysplasia and urinary infection in combination with renal failure lead to disability in patients with primary megaureter.
PERSPECTIVES AND DISCUSSION
As with all reconstructive surgeries, restoration of anatomical structures is necessary to obtain excellent functional results. The search is underway to reduce the morbidity of surgical intervention.
New endoscopic methods for treating this defect are being introduced, the influence of minimally invasive surgery and the use of laparoscopic techniques in reconstructive surgery are increasing.
BIBLIOGRAPHY
- Baskin LS, Zderic SA, Snyder HM: Primary dilated megaureter: long-term followup. J Urol 1994 Aug; 152(2 Pt 2): 618-21[Medline].
- Belman AB: Megaureter. Classification, etiology, and management. Urol Clin North Am 1974 Oct; DA - 19750131(3): 497-513[Medline].
- Creevy CD: The atonic distal ureteral segment (ureteral achalasia). J Urol 1967 Mar; 97(3): 457-63[Medline].
- Hendren WH, Henderson BM: Recent advances in pediatric surgery. Am J Surg 1969 Sep; DA - 19691031(3): 338-55[Medline].
- Lockhart JL, Politano VA: Management of massively dilated ureters in children. Urology 1981 Sep; DA - 19811118(3): 229-34[Medline].
- McLaughlin AP 3d, Pfister RC, Leadbetter WF: The pathophysiology of primary megaloreter. J Urol 1973 May; 109(5): 805-11[Medline].
- Rabinowitz R, Barkin M, Schillinger JF: The influence of etiology on the surgical management and prognosis of the massively dilated ureter in children. J Urol 1978 Jun; 119(6): 808-13[Medline].
- Rabinowitz R, Barkin M, Schillinger JF: Surgical treatment of the massively dilated primary megaureter in children. Br J Urol 1979 Feb; 51(1): 19-23[Medline].
- Rabinowitz R, Barkin M, Schillinger JF: Surgical treatment of the massively dilated ureter in children. Part II. Management by primary reconstruction. J Urol 1977 Sep; 118(3): 436-9[Medline].
- Tanagho EA: Embryologic basis for lower ureteral anomalies: a hypothesis. Urology 1976 May; DA - 19760802(5): 451-64[Medline].
- Williams DI, Hulme-Moir I: Primary obstructive mega-ureter. Br J Urol 1970 Apr; 42(2): 140-9[Medline].
eMedicine Journal, June 22 2001, Volume 2, Number 6 © Copyright 2001, eMedicine.com, Inc.
Indications for surgical urinary diversion
- Significant renal dysfunction
- Marked dilatation of the ureter and inhibition of its contractility
- Intractable secondary pyelonephritis
- Impossibility of performing radical surgery on a newborn due to the severity of the condition
Advantages
- Minimally invasive puncture installation
- Possibility of use in emergency cases in serious condition
Flaws
- Difficulty in care and the need to fix the tube
- Presence of a foreign body (supports pyelonephritis)
- Traumatization of the CLS
- Inadequate drainage of the middle/distal ureter
Advantages
- Does not require prompt closure
- Minimal trauma during surgery
Flaws
- Difficulty in care and the need to fix the tube
- Inadequate drainage of the middle/3, distal parts of the ureter
Advantages
- No trauma to the pelvis
- Good drainage of the CLS and the third ureter
- Possibility of long-term urine diversion
Flaws
- Inadequate drainage of the lower segment of the ureter
- decreased bladder volume
Advantages
- No trauma to the pelvis
- Good drainage of the urinary tract and upper segment of the ureter
- The possibility of long-term urine diversion with a bilateral process avoids the effect of “dry urinary tract”
Flaws
- Inadequate drainage of the lower segment of the ureter
- Decreased bladder volume
Advantages
- Rapid restoration of the size of the dilated ureter
- Easy to care for
- Possibility of long-term abduction
Flaws
- Invasiveness of the method
- Decreased bladder volume
- Limited use in bilateral process
- Scar processes that complicate radical surgery
- A comprehensive examination using functional and pharmacodynamic tests allows you to determine the degree of renal dysfunction, ureteral contractility and distinguish organic obstruction from functional
- We consider the following signs of organic obstruction: an obstructive type of curve on ultrasound with lasix loading, signs of vicarious hypertrophy of a healthy kidney, a gradual decrease in kidney function and contractility of the ureter against the background of progressive ureterohydronephrosis
- Identification of functional, compensated and decompensated variants of the course of obstructive megaureter allows us to accurately determine treatment tactics
- Functional obstructive megaureter is caused by neurogenic dysfunction of the bladder, is well treated conservatively and does not require surgery.
- In case of compensated megaureter with preserved renal function and good contractility of the ureter, radical surgery is indicated within 3-4 months. No preliminary urine diversion is required.
- For patients with decompensated obstructive megaureter with reduced renal function, in combination with bladder outlet obstruction, with depressed contractility of the ureters, and recurrent pyelonephritis, urinary diversion is indicated.
- Radical treatment is very dangerous, and often simply impossible. Preliminary surgical diversion of urine makes it possible to stabilize the condition of children in this group.
- For long-term urine diversion in bilateral lesions with a complicated course, T-shaped reverse ureterocutaneostomy and terminal ureterocutaneostomy in unilateral cases are most suitable.
Terminal ureterocutaneostomy is contraindicated in cases of megaureter of a single kidney.
Fig.1. Child 4 months with bilateral obstructive megaureter.
Fig.2. The same child at the stages of treatment at the age of 11 months.
Fig.3. The same child aged 2 years. There are no signs of megaureter, PMR is not detected - recovery.
from the website of Yu.E. Rudin www.drrudin.ru,
Is it necessary to examine the baby after birth?
In many children, moderate pyelectasis disappears spontaneously as a result of the maturation of the urinary system after the birth of the child. For moderate pyelectasis, it may be sufficient to conduct regular ultrasound examinations every three months after the birth of the child. If a urinary infection occurs, antibiotics may be necessary. As the degree of pyelectasis increases, a more detailed urological examination is necessary.
In cases of severe pyelectasis , if the dilation of the pelvis progresses and a decrease in kidney function occurs, surgical treatment . Surgery can remove the obstruction to the flow of urine. Some surgical interventions can be successfully performed using endoscopic methods - without open surgery, using miniature instruments inserted through the urethra.
Minimally invasive treatment of ureterocele in newborns
I.B. Osipov, R.A. Tee, D.A. Lebedev, A.I. Osipov Federal State Budgetary Educational Institution of Higher Education "St. Petersburg State Pediatric Medical University" of the Ministry of Health of the Russian Federation (St. Petersburg)
A ureterocele is a pathological intravesical formation, which is an enlarged intramural section of the ureter in the form of a cyst, which is usually caused by obstruction of the orifice and is accompanied by ureterohydronephrosis. An orthotopic ureterocele occurs in a non-duplicated ureter, the opening of which is located in the usual place; an ectopic ureterocele always corresponds to the accessory ureter of a duplicated kidney. Depending on the urodynamic disorders of the upper urinary tract, three degrees of ectopic ureterocele are distinguished. Depending on the degree of ectopia of the accessory orifice, the ureterocele can be cystic, urethral, or prolapsed. The latter often causes bladder outlet obstruction. The frequency of occurrence is 1: 3500 newborns, the ratio of boys to girls is 1: 4.
Antenatal diagnosis of ureterocele is based on the use of sonography. The sensitivity of the method ranges from 60 to 80%, diagnosis is possible from the 16th week of gestation. Diagnostic markers are the presence of a cystic formation in the lumen of the bladder, dilatation of the ureter and collecting system of the kidney, as well as oligohydramnios in case of complications with bladder outlet obstruction. After the birth of a child, pathology can be detected through ultrasound screening, which visualizes an intravesical anechoic formation. Among radiocontrast research methods, the leading role is given to excretory urography, which makes it possible to determine the filling defect of the bladder corresponding to the ureterocele, as well as a dilated ureter and an expanded pyelocaliceal system of the affected kidney or its accessory segment.
The indication for early surgical treatment of ureterocele is the presence of obstructive syndrome. The ureterocele is dissected at the base to form a free outflow of urine from the ureter. Operations can be performed using the open method with additional anti-reflux protection according to Gregoire. The operation of choice, in our opinion, regardless of the age group, is endoscopic correction. The latter is performed using diathermocoagulation or a high-energy laser while filling the bladder with a solution of 5% glucose. The criterion for the effectiveness of the neoostium is the ability to pass a 9 Ch cystoscope through it. In the case of preserved renal function, dissection of the ureterocele is a method of radical correction. In the absence of function of the kidney segment, this operation is regarded as the first stage of surgical correction of the malformation with the aim of decompression of the urinary tract and prevention of recurrent infection. If the function of the affected segment does not improve within 6–12 months, heminefroureterectomy is performed as a second step.
During the period from 2021 to 2021, 13 children with this developmental defect were operated on in the neonatal pathology department of the Perinatal Center of St. Petersburg State Pediatric Medical University. Of these, 8 children were operated on in the neonatal period and 5 children aged 2 to 3 months. The average age at the time of surgery was 31 days of life. By nature, 9 ureteroceles were ectopic and 4 were orthotopic. In all cases, endoscopic correction of the defect was effective.
Children with congenital ureterocele are subject to early surgical treatment in order to relieve the renal collecting system and prevent further progressive damage. Minimally invasive intervention is the operation of choice in newborns, regardless of the type, nature and degree of ureterocele. In the future, all children, after correction of the defect in the early period, are subject to mandatory dynamic observation and control examination.
Topics and tags
Pediatric urology
Magazine
Urological bulletins 2021 No. 1 - Special issue
Comments
To post comments you must log in or register