Congenital glaucoma is a disease in which intraocular pressure increases due to hereditary or congenital disorders in the structures of the eye through which intraocular fluid normally flows.
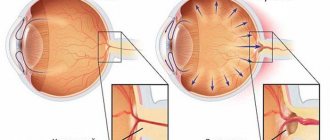
Normally, the intraocular fluid provides metabolic processes and maintains the required pressure inside the eye. It is formed during the filtration of blood from the capillaries of the ciliary body, part of the choroid of the eye, which is also involved in changing the shape of the lens, and flows mostly through a complex system of channels located in the corner of the anterior chamber. In this way, the constancy and regulation of intraocular pressure is maintained.
The anterior chamber angle is limited by the iris, ciliary body and cornea. The canal system is represented by the so-called trabecular meshwork, a system of thin membranes of different sizes that filter the intraocular fluid, which then enters the helmet canal - a narrow canal located inside the sclera, close to the cornea. From the Helmet canal, intraocular fluid flows back into the bloodstream.
Congenital glaucoma most often occurs in both eyes. The first signs of the disease appear already in the first 6-12 months of life. At the same time, unfortunately, if the disease is not detected in time and timely treatment is not carried out, up to 50% of children become blind by the age of 5-7 years.
Congenital glaucoma can be primary or secondary.
Clinic of congenital glaucoma
When symptoms appear early, the disease is most severe and has an unfavorable prognosis.
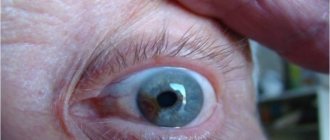
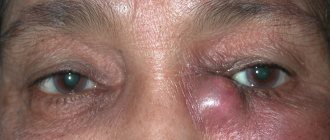
In children with congenital glaucoma, the first thing that attracts attention is the large and expressive (in the initial stages) eyes
The clinical symptoms of hydrophthalmos are influenced by the fact that the tissues of the child’s eye are easily extensible, and therefore changes occur in all its structures.
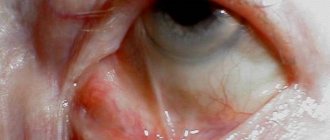
First signs The initial symptoms of hydrophthalmos are a slight enlargement of the cornea, the appearance of cracks in Descemet's membrane, and initially transient and then persistent corneal edema.
As the disease progresses, the cornea continues to stretch, the sclera becomes thinner, acquires a bluish tint (the choroid becomes visible), the limbus noticeably widens and the anterior chamber deepens.
Corresponding transformations also occur with the iris. Atrophic processes begin to develop in it, affecting the sphincter of the pupil. As a result, it expands and reacts sluggishly to light.
The lens usually has normal dimensions, but is flattened and moves back as the anterior chamber deepens. With a significant increase in the size of the eyeball, rupture of the stretched and thinned ciliary ligaments may occur, accompanied by subluxation or dislocation of the lens.
In an advanced stage of the disease, it often becomes cloudy (cataracts develop). The fundus is initially unchanged, but then glaucomatous excavation of the optic nerve begins to develop quite quickly. At the same time, the retina stretches and thins, which in the future can lead to its detachment.
In the early stages of the disease, IOP increases slightly and periodically, and subsequently it becomes persistent.
The progression of the disease leads to a steady deterioration in visual functions, primarily central and peripheral vision. At the onset of the disease, a decrease in visual acuity is caused by corneal edema.
Subsequently, vision deteriorates due to atrophy of the optic nerve, which manifests itself as glaucomatous optic neuropathy.
For the same reason, a threshold decrease in light sensitivity occurs in the paracentral and peripheral parts of the retina, which leads to specific changes in the field of vision of the affected eye.
In terms of shape, all congenital glaucoma, both hereditary and intrauterine, are classified as angle-closure. However, the reasons for obstructing the outflow of intraocular fluid are different, which allows us to distinguish two main clinical types of the disease - A and B.
Symptoms.
The disease usually progresses unnoticed.
In the first 2-3 months, parents may be alarmed by the child’s restless behavior, poor sleep and appetite; such children avoid bright light and may experience slight tearing.
The difficulty lies in the fact that the development of the child’s eye and brain occurs gradually from birth, so that only from 2 months the child has vision of the most insignificant quality, seeing only the silhouettes of objects and people.
So the decrease in visual functions, which suffer from glaucoma: visual acuity and peripheral visual field, cannot be determined during this period.
The severity of symptoms depends on the amount of intraocular pressure. With a significant increase in eye pressure, the eyeball increases in size, stretches, the cornea becomes cloudy, swollen, and the sclera, on the contrary, becomes thin due to stretching and acquires a blue tint.
Symptomatic manifestations and causes of pathology
Congenital glaucoma in children, even with the use of various methods of complex - conservative (medicinal) and surgical treatment - is practically incurable. This form of the disease is most often hereditary or caused by injury to the child in the womb or during childbirth.
Important! Congenital glaucoma is a disease that can make a child disabled; The sooner a diagnosis can be made and treatment carried out, the greater the chance the child will have for a normal, healthy and fulfilling life.
You can notice the first signs of the disease in the first week of a child’s life, but sometimes they appear only after several months, or even years.
Anatomically, the cause of glaucoma is explained by the abnormal development of the fluid outflow system inside the eyeball, which impedes the movement of intraocular fluid, which increases pressure.
Increased pressure in the eye entails changes in the eyeball itself - stretching of the cornea and sclera. This further leads to disruption of the supply of the optic nerve and complete blindness.
The reason for the occurrence and development of congenital glaucoma in the newborn lies in the pathological conditions of the mother during pregnancy, especially in its first trimester:
- Various infections and diseases suffered by the expectant mother: mumps (mumps), rubella, polio, syphilis, vitamin A deficiency;
- Food or any other poisoning;
- The influence of bad habits: smoking, and especially alcohol abuse;
- Hypoxia in the womb, as well as pathologies of the development of the nervous, endocrine and cardiovascular systems;
- Radiation and exposure.
With congenital glaucoma in children, symptoms manifest themselves as follows:
- The baby is born with large, “expressive” eyes, and subsequent enlargement of the eyeball progresses quite quickly;
- There is a rapid increase in the diameter of the cornea up to 20 mm, its clouding and swelling develops;
- Intraocular pressure increases;
- A slowdown in pupil reactions appears, photophobia and lacrimation develop, the child constantly blinks, and the eyes often turn red.
Congenital glaucoma is always accompanied by the development of pathological processes in other organs: the child may develop microcephaly, deafness, or heart disease. Diseases such as cataracts and aniridia may appear in the eyes.
In addition to congenital glaucoma, children also have secondary and infantile glaucoma.
Secondary glaucoma develops in the first months after the birth of the baby and is distinguished by the symptoms of the disease: there is no photophobia and lacrimation, the size and shade of the affected eye are practically no different from the healthy one.
The infantile (in other words, juvenile or juvenile) form occurs in children over 3 years of age and can develop in a person under the age of 35.
The causes of the development of this form of the disease are divided into three groups:
- Aging - the structure of the iris changes without changing the limbus, sclera and membrane; fluid pressure in the eye increases and vision deteriorates sharply;
- Pathology of the anterior region of the eye, which may be congenital. Symptoms are practically absent; diagnosis can only be made using a special examination;
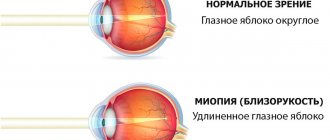
- Glaucoma occurs due to developing myopia - myopia.
The manifestations of signs of glaucoma development depend on the stage of the disease and its form: at the initial stage, the vitreous body of the eye is transparent, as the disease progresses, single opacities appear on it, at later stages multiple diffuse opacities and hemorrhages occur.
Blood circulation is disrupted, which leads to dystrophic changes in the optic nerve head, the development of its atrophy and subsequent blindness of the entire eye.
Treatment of juvenile glaucoma
Treatment begins with a conservative method, prescribing both local drugs and general agents. In the future, if the results are unsatisfactory or if the disease progresses rapidly, surgical treatment is resorted to.
Conservative treatment
Symptoms of the disease
Glaucoma can be found in the literature as “green cataract” or “dropsy of the eye,” which most accurately characterizes the main manifestation of the pathology.
Visible changes are characterized by clouding of the cornea, while it acquires an azure hue, reminiscent of green water. In children, the eyes acquire a special shine, and due to the protrusion of the cornea, the symptom of “cow eyes” appears.
Due to the protrusion of the cornea, the iris also suffers: sometimes there is atrophy along the inner edge and a change in color.
Infants with congenital pathology are very restless, they cry constantly. Decreased response to light. In parallel, other developmental anomalies are often detected: microcephaly, heart defects, deafness. It may also be observed:
- Aridia – absence of the iris;
- Microkernea is an unformed, small cornea.
In this case, the child experiences photophobia, accompanied by lacrimation. Redness of the sclera and their injection are often noted. Most often the process is two-way.
The child may complain of a cloudy picture or darkening of vision on a clear sunny day.
More often the process is chronic with periodic exacerbations. During exacerbations, rainbow circles appear when looking at the source of light rays, as well as a nagging pain in the eye, sometimes simulating a migraine attack, and sometimes even toothache.
Stages
Regular measurements of intraocular pressure can detect the early stage of the disease. It is recommended to carry out this procedure with the following frequency:
- at 35–40 years old - once a year;
- after 50 years and older - twice or thrice within 12 months.
If any abnormalities are detected, the specialist begins to collect an anamnesis, asks the patient questions regarding existing chronic diseases, previous head injuries, and the presence of relatives suffering from glaucoma. Next, the doctor conducts an ophthalmological examination, consisting of a detailed examination of the fundus of the eye, tonography (study of the outflow of intraocular fluid), perimetry, which examines the visual fields, and gonioscopy, which determines the condition of the anterior chamber of the eye.
There are 4 stages (degrees) of development of the disease. The prognosis is most favorable for the first degree of glaucoma. The early stage is characterized by minimal narrowing of visual fields and mild severity of other symptoms. At stages 2 and 3, the field of vision narrows significantly. At degree 4, a person expects complete blindness, which cannot be treated with surgery or other types of treatment. Therefore, the main task of doctors is the early diagnosis of glaucoma.
Comprehensive diagnosis of any form of glaucoma includes:
An ultrasound examination of the eyes is also necessary.
One of the most important studies is ocular tonometry - a procedure for measuring intraocular pressure. The doctor monitors exactly how the level of pressure changes when exposed to the cornea. The procedure can be carried out contact or non-contact. For contact tonometry, preliminary anesthesia is necessary.
The doctor also conducts:
- gonioscopy – examination of the anterior chamber of the eye;
- direct ophthalmoscopy - examination of the fundus.
Diagnostic measures aimed at confirming existing glaucoma and determining its type include:
- Daily tonometry (monitoring of intraocular pressure).
- Perimetry and campimetry aimed at identifying central and paracentral visual defects and narrowing the view.
- Direct or indirect ophthalmoscopy, biomicroscopy, which serves to detect changes in the fundus (at the initial stage of the disease, its condition often remains normal, but pathology can manifest itself as a shift in the vascular bundle in the area of the optic nerve).
- Ultrasound, electrophysiological studies.
To carry out a diagnostic examination, the child is often placed in a hospital department and the necessary manipulations are performed using anesthesia.
Definition of disease
Glaucoma is a chronic disease caused in most cases by increased intraocular pressure. Low and normal pressure is also observed in glaucoma. It manifests itself in the form of acute attacks, which become more frequent as the disease progresses. Normally, ocular fluid fills the anterior and posterior chambers of the eye.
Intraocular fluid contains the following useful substances:
- Ascorbic acid (vitamin C);
- Glucose.
Depending on the cause of glaucoma, various forms of the disease are distinguished. There are several classifications.
Classification by development mechanisms:
- Open-angle form of glaucoma. The corner of the eye is open. However, the spaces between the fibers of the pectineal ligament, which covers the canthus, are reduced. Aqueous humor does not penetrate the ligaments and is retained in the chambers of the eye. Occurs in 9 out of 10 cases of glaucoma.
- Primary open angle. Associated with direct pathology of the trabeculae of the pectineal ligament. There are various reasons for the development of this form of the disease. The main one is age-related changes after 35 years. Characterized by an asymptomatic course. The anatomical features of the eye structure increase the likelihood of developing the disease.
- Exfoliative open angle. The reasons for its appearance are disturbances in biochemical processes in the connective tissue of the body. The by-product in the form of layers (exfoliant) settles on the pectineal ligament and prevents the outflow of fluid. Occurs in adults.
- Pigmented open-angle. The pigment exfoliated from the iris clogs the trabeculae of the ligament. Aqueous humor does not drain from the eye. It occurs at a young age, unlike other forms of open-angle glaucoma.
- Angle-closure form of glaucoma. It occurs in the form of attacks, which are provoked by drops that dilate the pupil and insufficient lighting. The reasons are dilation of the pupil, which causes thickening of the root of the iris. The corner of the eye closes. The block prevents the outflow of fluid. More often develops in the presence of farsightedness. A large lens and a narrow angle of the eye from birth is what causes angle-closure glaucoma.
Classification by reason of occurrence:
- Primary. An independent pathology that is not associated with damage to other body systems;
- Secondary. Develops secondary to other diseases and medications.
Classification by age of onset:
- Congenital primary. Occurs in children under 2 years of age without provoking causes. Occurs less frequently than in adults. Caused by a violation of the development of the anterior chamber angle. You can suspect it by photophobia and constant lacrimation. During examination, the ophthalmologist reveals an enlarged eyeball.
- Infantile primary. Develops in children from 3 to 11 years old. The pathogenesis is the same as that of congenital glaucoma.
- Juvenile primary. From 12 to 36 years. The same development mechanism.
- Glaucoma in adults. The most common form.
Classification
Congenital glaucoma is classified into types in clinical practice depending on its causes.
Varieties
- Primary, caused by genetic disorders,
- Secondary, developing due to impaired development of the visual organs,
- Combined, which is characterized by both hereditary conditions and acquired problems.
Glaucoma is divided into 3 degrees
Primary congenital glaucoma is classified into the following clinical grades:
- early - it manifests itself before the age of three years,
- infantile - symptoms develop between the ages of 3 and 10 years,
- juvenile - it manifests itself most often only during adolescence.
The form of primary congenital glaucoma depends on the degree of underdevelopment of the ocular structures responsible for drainage of fluid.
Glaucoma in children - types of disease
Glaucoma is one of the most dangerous diseases in ophthalmic practice. It is characterized by an increase in pressure in the chambers of the eye due to disruption of the outflow of intraocular moisture. The disease most often manifests itself, of course, in patients over 45-50 years of age, but, nevertheless, it can often be diagnosed in children.
In childhood, pathology can be divided into the following types:
Congenital
The disease is diagnosed in children under one year of age. It is believed that the main reason is hereditary predisposition. However, traumatic damage to the organ of vision during the passage of the child through the mother’s birth canal or damage to the embryo in the prenatal period are also distinguished.
The cause of intrauterine damage can be either an infectious disease of the mother or the influence of trigger factors: smoking, alcohol, drug addiction, poisoning, taking medications, especially in the first trimester, when the development of the organ of vision in the unborn child occurs.
Infantile
It occurs in children from two to ten years old. The cause may be congenital defects that manifest themselves later.
Juvenile or juvenile glaucoma
Most often it is secondary and is the result of acquired diseases, diagnosed in children after 10 years.
It can also be transmitted hereditarily, is a dominant trait, and is more common in boys. Accordingly, children can have both congenital and acquired glaucoma. The disease can also be primary, that is, develop directly as a result of eye pathology or anatomical defects.
Glaucoma can also be secondary: against the background of eye myopia, trauma, against the background of pathologies of other systems and organs, infectious lesions, and so on.
Complications and prognosis
In the postoperative period, local anti-inflammatory, antibacterial and metabolic drugs are prescribed. If complications occur, vasoconstrictors and hemostatic agents are first prescribed.
After surgery, you should continue to visit your ophthalmologist to monitor the results of your treatment. IOP readings need to be checked three times a year. From the age of 15, in the absence of alarming signals, tonometry is performed annually during a routine examination by an ophthalmologist.
Adverse factors after surgery:
- Fibroblast proliferation and excessive scarring. In children, such phenomena are observed more often. For this reason, fistulizing techniques are less effective in the fight against congenital glaucoma. Insufficient intervention and the formation of goniosynechia after hemorrhage can contribute to the formation of scars. It is possible to reduce proliferation with the help of glucocorticosteroids, cytostatics and proteolytic enzymes.
- Anterior chamber reduction syndrome. The condition develops with a strong outflow of moisture through a new channel, poor regeneration and external filtration. The syndrome is expressed in a decrease in the space between the cornea and the iris, an increase in IOP and a decrease in total blood pressure.
- Ciliochoroidal detachment. The main causes of detachment: decompression, shift of the ciliary body, hypotension, change in blood movement through the capillaries. Ciliochoroidal detachment is a characteristic complication of Sturge-Weber syndrome.
- Hyphema (hemorrhage in the anterior chamber), hemophthalmos (blood entering the vitreous body). Such complications are treated by using vascular strengthening, hemostatic, antioxidant and anti-inflammatory drugs. It is possible to prescribe mydriatics.
The success of treating congenital glaucoma depends on how quickly the increase in IOP is detected and how severe it is. After surgery, the prognosis is usually favorable. If the procedure is carried out on time, vision is restored in 75% of patients. Delayed treatment becomes more difficult, severe complications appear more often, and the number of successful operations decreases to 25%.
Diagnosis of glaucoma
Most older people consult a doctor with red eyes and limited vision - these two symptoms are present in all patients. If a person also complains of spots and “rainbows” around light sources, then his condition should be distinguished from migraine, which manifests itself in a similar way.
The most accurate diagnosis is considered to be a combination of various methods of tonometry and perimetry. Glaucoma and surgery for its treatment require a multifaceted approach to the disease.
Methods for measuring intraocular pressure
This group of methods includes tonometry and elastotonometry.
In order to determine the pressure inside the eye using tonometry, the patient must take a supine position. The measurement is carried out with a ten-gram Maklakov tonometer. When identifying glaucoma in the early stages, it should be taken into account that IOP changes throughout the day and depends on many factors: breathing, pulse fluctuations, changes in vascular tone, etc. In a person with incipient glaucoma, the range of these changes will be greater than in healthy people. Therefore, the so-called daily tonometry is carried out: the measurement is carried out early in the morning, even before getting up, and also 12 hours later. The difference between these measurements should normally not be more than 5 mmHg. Art.
You can measure the pressure inside the eye using other methods. For example, Goldman tonometry or the elastotonometry method. To carry out the latter, sets of tonometers are used that differ from each other in weight: for example, the Filatov-Kalf elastotonometer is a set of Maklakov tonometers with weights of different weights. For a healthy person, the elastotonometric curve obtained as a result of such an examination will be almost straight.
The most accurate method for diagnosing glaucoma is electronic tonography, which requires a specialized device - an electronic tonograph.
Method for measuring the outflow of intraocular fluid
Gonioscopy methods can be used to assess the condition of the drainage system. During the procedure, using a gonioscope or goniolens, the condition of the anterior chamber angle is analyzed.
This method makes it possible to assess the anatomy of the patient’s organ of vision and identify his tendency to develop angle-closure glaucoma. In addition, this diagnostic method is useful when choosing the type of surgical operation.
Perimetry
Analysis of the boundaries of the visual field is called perimetry. Due to its informative content, it allows not only a high-quality diagnosis of glaucoma, but also makes it possible to assess how effectively the patient’s treatment is progressing. Most often prescribed:
- isopperimetry – analysis of field boundaries, which uses objects of different areas;
- campimetry – analysis of damage to the central visual field.
During clinical observation of a person with glaucoma, static and kinetic types of perimetry are also used. In particular, computer perimetry may be prescribed, which is usually performed once every 3 months.
Diagnosis of the optic nerve condition
To determine the degree of damage to the optic nerve, ophthalmoscopy is performed - this is the name of the method for diagnosing glaucoma, during which the doctor examines the fundus of the eye.
If it is necessary to conduct a better diagnosis of the optic nerve, the following are prescribed:
Optical coherence tomography.
At its core, the method is close to ultrasound, but to study tissues, not acoustic waves are used, but near-infrared radiation with a wavelength of about 1 micron. So it would be more accurate to call it not tomography, but echo sounding.
Confocal scanning laser ophthalmoscopy.
A method that allows you to obtain a three-dimensional image of the optic nerve head.
Laser polarimetry.
This test measures the width of the retinal neuron layer by applying a polarized light effect.
Diagnostics.
If you have the slightest suspicion of congenital glaucoma, you should immediately consult an ophthalmologist. Doctor:
- will check the visual functions of the eye, at least approximately.
- will assess the level of intraocular pressure - in young children it is checked with light finger pressure, that is, by palpation. With the help of devices it is measured in a state of sleep.
- will examine the eye under a microscope in order to establish the correct diagnosis and, if necessary, prescribe treatment.
Symptoms of Glaucoma in children:
Typical symptoms of glaucoma in children:
- photophobia, lacrimation
- increased IOP
- optic disc changes
- increase in corneal diameter and limbal width
- an increase in the size of the eyeball that progresses rapidly
- slowing of pupillary reactions
- corneal edema
With congenital glaucoma, children may have defects in other organs and systems:
- heart defects
- microcephaly
- phakomatoses
- deafness
With congenital glaucoma, there may be other pathological processes in the eye:
- cataract
- aniridia
- microkernea
In most cases, congenital glaucoma occurs in both eyes. Often the child has no complaints, except those caused by corneal syndrome. This means that the disease proceeds like open-angle glaucoma. Late stages of the disease are manifested by the appearance of staphylomas, sometimes ruptures of the sclera, there may be stretching and thinning of the conjunctiva, complicated cataracts. At the onset of congenital glaucoma, the fundus is normal.
With the congenital form of glaucoma, the child’s visual functions deteriorate, since first changes in the cornea and refractive errors occur, and then damage to the optic nerve and retina occurs.
Syndromes involving childhood glaucoma
With some diseases, in some cases the child develops glaucoma.
Sturge-Weber syndrome (facial angioma)
With this syndrome, intracranial angiomas, purple facial telangiectasias and glaucoma appear. It is diagnosed in a third of patients with this syndrome. Glaucoma can be detected while the child is very young. But there are many cases where manifestations of glaucoma began in children of preschool and school age.
Marbling of the skin in congenital telangiectasia
This is a rare syndrome that is similar to the one described above. Vascular disorders appear due to skin damage. The child has marbled skin, apoplexy, sometimes convulsions and glaucoma.
With this disease of the first type there may be glaucoma. It is often combined in these cases with plexiform neuromas of the orbit and ipsilateral coloboma of the iris or eyelid. The reasons lie in the pathology of the tissues of the anterior chamber angle or the closure of the angle, which is caused by neurofibromatosis.
Rubinstein-Taybi syndrome
It is extremely rare and is characterized by an anti-Mongoloid eye shape, hypertelorism, wide fingers, elongated eyelashes, and enlarged toes. Presumably, glaucoma results from underdevelopment of the anterior chamber angle.
Peters anomaly
This is a central clouding of the cornea that occurs in a child from birth; the eye is combined with defects in Descemet's membrane, stroma and endothelium.
Juvenile open angle glaucoma
This form of glaucoma is rare and is inherited in an autosomal dominant manner. Clinical examination does not provide the doctor with the necessary information. Histological examination is effective as a diagnostic method.
Secondary glaucoma
Pathology of the lens and its interaction with the iris diaphragm
With spherophakia, there is a tendency for the lens to shift forward and the appearance of glaucoma. The disease can occur in isolated spherophakia and in Weill-Marchesani syndrome.
Aphakic glaucoma
The disease may begin several years after cataract extraction. The pathogenesis remains a complete mystery, but in some cases it is associated with the development of pathological changes in the angle of the anterior chamber. The prognosis is unfavorable.
Retinopathy of prematurity
Glaucoma can occur with severe retinopathy of prematurity, when total damage to the retina occurs.
Juvenile xanthogranuloma
This disease often occurs as a cutaneous process, and more rarely as an intraocular process, which causes glaucoma (it usually appears due to hemorrhages).
Glaucoma in inflammatory eye diseases
The disease in question may be a consequence of uveitis. Treatment consists of suppressing inflammation. In some cases, glaucoma can be caused by blockage of the trabecular meshwork by exudate and acute trabeculitis.
Injury
Blunt trauma to the eyeball (hyphema, angle recession) can also cause glaucoma in children.
Surgery
Urgent operations are carried out at a critical level of eye pressure, which could not be reduced in any other way. Surgical treatment is recommended even when IOP levels were initially low, but medications did not bring results.
Surgical techniques for eliminating congenital glaucoma:
- Restoring the outflow of ocular moisture by eliminating defects in the natural course of the drainage network or by forming a new course (fistula).
- Reducing the production of intraocular fluid.
With a congenital increase in IOP, the venous sinus of the sclera remains functional for a long time. Since the outflow defect is located inside the sinus, it is better to choose reconstructive microsurgical operations at an early stage. This intervention allows you to form a channel for excess fluid to flow to the sinus.
Restoring outflow
All methods of forming normal outflow of ocular fluid are divided into two groups. The first includes methods that eliminate defects in the natural drainage path, and the second includes fistulizing operations that form additional channels.
Elimination of defects in the drainage network of the eye:
- Goniotomy is the dissection of adhesions in the chamber angle, which allows to improve the outflow of fluid through the venous sinus of the sclera. The operation is indicated only for patients with a transparent cornea up to 3 mm. It is possible to additionally perform goniopuncture when a spare channel is provided under the conjunctiva.
- Trabeculotomy (ab interno) - making incisions in a section of the trabecular meshwork. The procedure can be performed at an early stage even if the cornea is clouded. The surgeon makes an incision under the conjunctiva and scleral flap, opening the venous sinus of the sclera. A very thin probe is placed into the incision and a gap is created in the wall of the sinus.
- Laser trabeculopuncture.
At the late stage of congenital glaucoma, when the cornea is severely damaged, it is recommended to resort to fistulizing and similar techniques. They make it possible to create alternate routes that will “unload” the main drainage network.
Fistulizing techniques:
- Diathermogoniopuncture, microdiameterogoniopuncture.
- Trabeculotomy (ab externo).
- Goniodialysis and trabeculotomy ab externo.
- Sinustrabeculectomy.
If the disease has developed greatly, they resort to combined techniques and create backup pathways under the mucosa and in the perichoroidal area. This is the space between the vessels and the sclera.
Combined techniques for reducing IOP:
- Trepanocyclogoniotomy and diathermocoagulation.
- Penetrative goniodathermy.
- Cyclodiametry.
- Sclerectomy with cyclodialysis or trabeculogoniotomy.
- Trabeculectomy and cyclostomy.
Reducing the amount of eye fluid
When the eyeball reaches a critical size, the risk of complications after abdominal surgery increases. It is much safer, instead of forming a new outflow, to reduce the production of ocular moisture. Such operations are based on working with the ciliary processes or the posterior ciliary arteries that feed them. Temperature is usually used. After the surgical procedure, the child is prescribed Acetazolamide for several days to enhance the effect on the functioning of the ciliary body.
Techniques for operating the ciliary processes:
- Cyclophotocoagulation.
- Cyclocryodestruction.
- Cyclocryopexy.
- Cyclodiathermy.
- Laser contact or non-contact cyclocoagulation.
Diathermocoagulation (cauterization) of arteries is considered only as an alternative. Such an effect significantly reduces blood flow to the ciliary topic, affecting its functionality.
Treatment of complicated glaucoma
Congenital glaucoma may be a complication of lens subluxation in Marfan syndrome. Only removal of the lens through phacoemulsification will help restore the normal outflow of ocular moisture.
Sometimes glaucoma is combined with angiomatosis, that is, excessive growth of the vascular network occurs. In these cases, cyclodiathermocoagulation or iridencleisis is indicated. When glaucoma is complicated by neurofibromatosis, iridectomy is performed. In the case of phakomatosis - wide or basal. The operation makes it possible to remove angiomatous and neurofibromatous growths in the area of the chamber angle.
What diseases are not allowed on the plane?
Having noticed signs of illness in a passenger, the airline administration has the right to refuse him boarding the aircraft.
Therefore, for those who are interested in the question “do they have the right not to let someone with chickenpox board a plane?”, I will answer as follows. Having discovered a pronounced rash on the body, an airline employee may ask for a certificate from a doctor confirming the non-infectious nature of the disease.
Causes
Why does eye glaucoma occur? Often the disease is caused by a group of factors. The main thing that causes the disease is intraocular hypertension syndrome.
Genetic reasons:
- If glaucoma is detected in close relatives, then the likelihood of developing the disease increases 2–4 times. Specific genes responsible for the occurrence of glaucoma have been identified;
- The anatomical features of the eye (small vitreous body, large lens, narrow angle of the anterior chamber of the eye) prevent proper outflow of fluid.
Anomalies of eye development:
Age-related changes in the eye:
- in adults, due to the occurrence of degenerative changes, the capacity of the pectineal ligament decreases;
Metabolic disorders:
- pseudoexfoliation syndrome (an exfoliant substance is deposited in the trabecular system);
- pigment syndrome (desquamated iris pigment clogs the corner of the eye).
Factors causing narrowing of the iridocorneal angle:
- farsightedness (focusing the eye on distant objects leads to the expansion of the lens, which closes the entrance to the angle between the cornea and the iris);
- the use of drops that dilate the pupil (a fold forms at the base of the iris, preventing the outflow of aqueous humor).
Extraocular causes:
- diabetes mellitus, which leads to retinopathy;
- taking steroid drugs.
Considering the causes of the pathological process, the disease is divided into several varieties. Experts distinguish primary, secondary and congenital forms of the disease, each of which is characterized by its own provoking factors.
Doctors have not identified the main reasons that provoke the development of glaucoma in children. Experts are inclined to believe that the disease can manifest itself due to hereditary predisposition or due to the influence of other factors while the child is in the womb.
The following prerequisites for the disease are identified:
- pathologies of the nervous, cardiovascular, or endocrine systems;
- infectious diseases of the mother during pregnancy (typhoid, rubella, polio, syphilis, mumps, toxoplasmosis, vitamin deficiency);
- abnormal structure of the child's eyes;
- mother's bad habits;
- hypoxia suffered by the fetus in the womb.
Causes of congenital glaucoma
The causes of the development of this form of the disease are divided into three groups:
- Aging - the structure of the iris changes without changing the limbus, sclera and membrane; Fluid pressure in the eye increases and vision deteriorates sharply.
- Pathology of the anterior region of the eye, which may be congenital. There are practically no symptoms; diagnosis can only be made using a special examination.
- Glaucoma occurs due to developing myopia - myopia.
Sometimes glaucoma in children can occur due to injury to the eyeball, after inflammatory and infectious eye diseases.
The manifestations of signs of glaucoma development depend on the stage of the disease and its form: at the initial stage, the vitreous body of the eye is transparent, as the disease progresses, single opacities appear on it, at later stages multiple diffuse opacities and hemorrhages occur.
Blood circulation is disrupted, which leads to dystrophic changes in the optic nerve head, the development of its atrophy and subsequent blindness of the entire eye.
The causes of this anomaly are various pathological conditions of the woman, especially in the first months of pregnancy. They are caused by a wide variety of reasons: infections (measles, rubella, influenza, etc.), poisoning, alcoholism, ionizing radiation, etc.
The reason for the occurrence and development of congenital glaucoma in the newborn lies in the pathological conditions of the mother during pregnancy, especially in its first trimester:
- Various infections and diseases suffered by the expectant mother: mumps (mumps), rubella, polio, syphilis, vitamin A deficiency.
- Food or any other poisoning.
- The influence of bad habits: smoking, and especially alcohol abuse.
- Hypoxia in the womb, as well as pathologies of the development of the nervous, endocrine and cardiovascular systems.
- Radiation and exposure.
Often:
- Primary congenital glaucoma. Not combined with other or systemic disorders. The diagnosis is made when other causes of glaucoma are excluded.
- Sturge-Weber syndrome. Usually the lesion is unilateral; there may be a port-wine stain, calcifications in the brain, and seizures; the disease is not familial. The incidence of glaucoma increases when hemangioma affects the eyelids and conjunctiva.
Rarely:
- Developmental anomaly of the anterior segment. Axenfeld syndrome, Rieger anomaly/syndrome, Peters anomaly, etc.
- Lowe's syndrome (oculocerebrorenal syndrome). Cataracts, glaucoma and kidney disease; X-linked recessive inheritance.
- Rubella. Glaucoma, salt-and-pepper chorioretinopathy, hearing impairment and cardiac disorders. Cataracts may also occur, but not in combination with glaucoma.
- Aniridia. Hypoplasia of the iris, often gonioscopy reveals only a rudimentary remnant of the iris, cataracts, glaucoma, foveal hypoplasia, nystagmus.
- Others (eg, neurofibromatosis, homocystinuria, primary persistent hyperplastic vitreous, secondary glaucoma due to anterior displacement of the iridolenticular diaphragm).
For congenital glaucoma in children, examinations are carried out in a medical genetic consultation to determine the stage of the disease and its cause. The doctor may also need a pregnancy chart to identify prerequisites for the occurrence of glaucoma.
In conclusion, I would like to note that childhood glaucoma is not a sentence to blindness, but only if the mother makes every effort to timely diagnose and treat the disease.
In addition, modern microsurgical techniques make it possible to cope with the disease and stop its development at an early stage. Therefore, our experts recommend that you be attentive to your child’s health and do not forget to regularly visit the ophthalmologist.
Glaucoma of childhood
Sidorenko EI Authors reveals the problem of pathogenesis, imperfection of classification and treatment of infantile glaucoma. The classification of infantile glaucoma is listed in details. Unfortunately, childhood glaucoma has significantly more problems than adult glaucoma. Even on the issue of classification of childhood glaucoma, there is no consensus; in pediatric ophthalmology, non-perforating types of operations - viscocanaculostomy, excimer types of surgery for glaucoma - are not widely used; gonioscopy is not widely used due to the negative attitude of children towards this procedure. Many research methods are more suitable for adults, require a certain intelligence and cannot be used in children. The classification described in the textbook by Kovalevsky E.I. is completely out of date and does not reflect the advances in glaucoma science. A more progressive classification was proposed by A.V. Khvatova in 1982, but it was never completed. In 1991, Sidorov E.G. together with Krasnov M.M. proposed a new classification. It came much closer to the classification of adults, but never found widespread use in clinical practice due to its cumbersomeness. In 2001, we proposed a classification that is closest to the classification of glaucoma in adults, since we are convinced that the violation of hydrodynamics in glaucoma in adults and children is based on the same reasons - dysgenesis of the anterior chamber angle. Severe dysgenesis of the anterior chamber angle is more severe and manifests itself immediately after the birth of a child - congenital primary glaucoma. Less severe dysgenesis of the anterior chamber angle manifests itself as juvenile primary glaucoma. And, finally, small changes in the anterior chamber angle, acting as nuances in the structure of the anterior chamber angle (A.P. Nesterov), are realized in primary glaucoma in adults. Glaucoma in children and adults caused by dysgenesis of the anterior chamber angle should be classified as primary glaucoma. Based on this concept, one should distinguish between primary (children and adults) and secondary glaucoma. Primary congenital glaucoma (PCG) Congenital primary glaucoma is one of the most severe eye pathologies in newborns, leading to early blindness. The disease is relatively rare - one case per 10,000 children, but among blind children, congenital glaucoma causes vision loss in 2.5-7% of cases, that is, almost every 10 child becomes blind from congenital glaucoma. The disease in 15% of cases is hereditary (familial) in nature or may be caused by disorders in the prenatal period and is more common in boys (3/2). In general, congenital glaucoma is a pathology of the first year of a child’s life, since by this age it manifests itself in more than 90% of cases. With congenital glaucoma, there are gross signs of dysgenesis in the anterior chamber angle, so it manifests itself early and progresses quickly, leading to irreversible vision loss within 2–3 weeks. Therefore, early diagnosis and early surgical treatment, aimed at eliminating obstacles to fluid flow and creating artificial outflow pathways, play a special role. Early diagnosis of the disease, which is crucial for successful treatment, is only possible with a good knowledge of its early signs by obstetricians, neonatologists and micropediatricians of maternity hospitals, local pediatricians and nurses caring for small children, since a pediatric ophthalmologist examines all children only at 2–4 one month of age in order to exclude congenital pathology. Etiology: 1. Hereditary glaucoma is observed in 15% of cases, is transmitted in an autosomal recessive manner and is often combined with other anomalies in the eye. 2. Intrauterine glaucoma develops under the influence of teratogenic factors (X-ray studies, hypoxia, vitamin deficiency, toxicosis, infectious diseases). Pathogenesis. We have already spoken partially about it. Congenital glaucoma is a consequence of incorrect and incomplete splitting of tissue in the anterior chamber angle during embryogenesis. Dysgenesis and anomalies of the anterior chamber angle in congenital glaucoma are pronounced. The main role, like primary glaucoma in adults, is played by retention (obstruction) of the outflow of intraocular fluid. Changes in the angle of the anterior chamber can be very diverse, up to its complete non-splitting and the absence of many links in the outflow tract. According to the localization of retention in congenital glaucoma, one should distinguish: – pretrabecular open-angle glaucoma, which comes in first place in terms of frequency of occurrence (62%). The angle is open, but mesodermal tissue is defined pretrabecularly; – pretrabecular angle-closure glaucoma (14.7%), which may be caused by the closure of the trabecula by the root of the iris, the ciliary body; – trabecular glaucoma, which can be caused by underdevelopment of trabeculae, sclerosis, its absence, pathological inclusion of ciliary muscle fibers in the trabecula; – intrascleral retention, which occurs in the absence, deformation, dislocation of Schlemm’s canal, with underdevelopment of the scleral spur, intramural outflow tracts, up to their complete absence. These gross changes in the drainage system leave no hope for success from therapeutic treatment and require urgent surgical intervention. Diagnosis of congenital glaucoma With congenital glaucoma, intraocular fluid is produced, the outflow of which is sharply hampered, fluid accumulates, and intraocular pressure increases. The load on the outer shell of the eye increases sharply and it begins to stretch. The child’s eyes at the beginning of the disease are beautiful: the anterior chamber deepens, the eyes are large, expressive, the sclera becomes bluish from stretching (the vascular sclera becomes visible). With continued stretching of the eyeball, its size sharply increases (buphthalmos - “bull’s eye”), the cornea becomes cloudy, the sclera sharply thins and in the form of staphylomas unevenly protruding outward, the eyes are blind, oscillating in nystagmus. The initial signs of congenital glaucoma are the horizontal diameter of the cornea slightly larger than the age norm. You can focus on the following dimensions: the diameter of the cornea in newborns is 9 – 9.5 mm; at 1 year – 10–10.5 mm; at 2–3 years – 10.5–11 mm. The depth of the anterior chamber in newborns is 1.5–2 mm; at 1 year – 2.5 mm; at 2–3 years – 3–3.5 mm. After 6 years, these data approach the size of an adult human eye - the diameter of the cornea is about 11.5 mm, the depth of the anterior chamber is about 3.5 mm; – the anterior chamber deepens; – with an increase in IOP, the limbus begins to stretch (expand), since in this place the outer shell is thinned and fragile. The limbus becomes wider than 1 mm; – the anterior ciliary vessels dilate; – the sclera stretches and the vascular tissue is visible through it – the sclera receives a delicate bluish tint; – a gentle (like morning fog) swelling of the cornea appears – opalescence. Stretching of the cornea leads to cracks in the endothelium and leakage of fluid into its thickness. Normally, in 15% of cases, newborns experience physiological opalescence of the cornea, which disappears within 1 week. For differential diagnosis, a 5% glucose solution or glycerin is instilled into the child’s eye - the pathological edema disappears, the physiological opalescence remains; – by 2–3 months, cranial innervation improves and photophobia appears due to irritation of the corneal nerves; – the pupil dilates and its sluggish reaction appears due to muscle atrophy; – a shift of the vascular bundle is noted in the fundus; – the eyes are large, beautiful, the diameter of the cornea increases, especially along its horizontal meridian. Secondary congenital glaucoma Secondary congenital glaucoma differs significantly from adult glaucoma in the variety of forms and is a consequence of other diseases. Secondary congenital ophthalmic glaucoma (SCOG) – with anomalies of the anterior segment of the eye 1) Aniridia in 50% of cases can be complicated by an increase in IOP. Glaucoma most often appears in adolescence. Children with aniridia should be under clinical supervision, with systematic monitoring of intraocular pressure. 2) Ectopic lens often results in the root of the iris being pressed against the trabecula or cornea by the displaced lens, causing glaucoma. 3) Rieger syndrome – mesodermal dysgenesis of the iris and cornea, a hereditary disease with a dominant type of transmission. The syndrome includes hypoplasia of the anterior layer of the iris, embryotoxon, mesodermal bridges extending from the basal part of the iris to the embryotoxon. Glaucoma usually develops after the first decade of life, and therefore the eyeball usually does not enlarge. 1) Frank-Kamenetsky syndrome is observed in men and is transmitted by a recessive, X-linked type. A distinctive feature of this iris hypoplasia is its two-colored appearance: the pupillary zone of 1.5–2 mm is grayish or blue or brown in color, the wider ciliary zone looks chocolate brown due to the exposure of the pigment layer. Glaucoma develops in the second decade of life. Secondary congenital syndromic glaucoma (SCSG) 1) Sterzh-Weber syndrome (encephalooculofacial hemiangiomatosis, encephalotrigeminal angiomatosis). The etiology is not fully understood, but heredity is of great importance. A systemic disease manifested by capillary hemangioma of the face, hemangioma and focal changes in the brain. A characteristic feature is capillary hemangioma of the face along the branches of the trigeminal nerve in the form of extensive purple spots. Neurological symptoms, which can manifest as epilepsy, hydrocephalus, paresis, mental disorders, and mental retardation, depend on the degree of intracranial angiomatosis. Glaucoma develops on the side of the nevus when the eyelids, especially the upper, and conjunctiva are affected. Such patients require systematic monitoring of IOP. 2) Neurofibromatosis. Congenital glaucoma can be observed in its generalized form with damage to the skin, bones, brain, endocrine system, and especially when localized in the area of the upper eyelid and temple. 3) Marfan syndrome, Marchesani - family-hereditary diseases, with damage to connective tissue, cardiovascular, musculoskeletal, endocrine and other body systems, accompanied by ectopia of the lens and, as a consequence, secondary glaucoma. Secondary acquired glaucoma (SAG) This type of glaucoma develops after diseases or injuries of the eye. The immediate cause of increased IOP in all forms of secondary glaucoma is a violation of the outflow of aqueous humor from the eye. A large number of different forms of secondary glaucoma can be combined into several groups: 1) uveal post-inflammatory glaucoma; 2) phacogenic glaucoma; 3) vascular glaucoma; 4) traumatic glaucoma; 5) glaucoma caused by degenerative processes; 6) neoplastic glaucoma. In uveal glaucoma, an increase in IOP is caused by the formation of goniosynechia, accumulation of exudate in the trabecular zone, fusion and fusion of the pupil. Anamnesis and detection of the described changes in the eye allows us to make a diagnosis. Phacogenic glaucoma occurs due to diseases of the lens: – phacotopic glaucoma is caused by a change in the position of the lens when it is subluxated or dislocated; – swelling of the cataract can also cause impaired hydrodynamics and increased ophthalmotonus – phacomorphic glaucoma; – overripe cataract causes phacolytic glaucoma due to the resorption of its masses and the macrophages involved in this close the trabecular membranes; Secondary vascular glaucoma often develops as a result of thrombosis of the central retinal vein, diabetes. Neovascularization in the angle of the anterior chamber impedes the outflow of fluid from the gas. Phlebohypertensive glaucoma occurs when pressure in the venous vessels of the orbit increases due to vascular tumors or compression of the superior vena cava. Traumatic glaucoma is divided into contusion and wound. In case of contusion from a hydrodynamic shock, the tabecular apparatus is often damaged and the angle of the anterior chamber is split, which complicates the outflow of fluid from the eye. Traumatic glaucoma is caused by adhesions in the angle of the anterior chamber. Degenerative secondary glaucoma occurs with uveopathy, phakomatoses, old retinal detachment, and with retinopathy of various origins. Neoplastic glaucoma develops from intraocular tumors, when their breakdown products obstruct the outflow of fluid from the eye.
Types of primary glaucoma
According to IOP indicators:
- with increased IOP;
- normotensive (with normal IOP values).
According to the dynamics of the disease:
- stabilized;
- unstabilized.
Stages:
- initial;
- developed;
- far gone;
- terminal.
According to the mechanism of disease development:
- Open angle. This variant of the disease is the result of dysfunction of the drainage system of the eye. This may occur due to deposition of iris pigment in the trabecular apparatus, changes in the structure of this apparatus, etc.
- Closed angle. With this type of disease, a violation of the circulation of aqueous humor through the pupil occurs, which leads to prolapse of the peripheral part of the iris into the area of the anterior chamber. In this case, the root of the iris blocks the angle of the anterior chamber, disrupting the circulation of aqueous humor.
- Mixed. Mixed variants of primary glaucoma are also common, when the patient has both pathogenetic mechanisms of the disease.
Determining the type of glaucoma is an important task, which is achieved using modern diagnostic methods at the Eye Surgery Center. Finding out the causes and mechanisms of the disease allows you to plan the most effective treatment tactics.