The structure and functioning of the adrenal glands
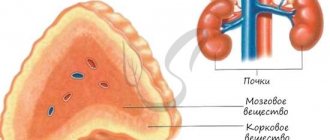
The adrenal glands are based on two structures:
- Brain matter.
- Cortical substance.
They are regulated by the nervous system.
Brain matter
The medulla is the main source of catecholamine hormones in the body - adrenaline and norepinephrine.
Adrenaline is the main hormone in the fight against stress. An increase in its production occurs during positive and negative emotions, for example, during injury or a stressful situation.
When exposed to adrenaline, the body uses reserves of accumulated hormone, which is manifested by the following reactions:
- a sudden surge of strength;
- increased breathing;
- pupil dilation.
The person feels stronger than usual, and the pain threshold is significantly reduced.
Norepinephrine is also a stress hormone, but its production occurs before the production of adrenaline. The impact is much weaker. Its function is to regulate blood pressure, which stimulates the work of the myocardium - the heart muscle.
Cortex
The adrenal cortex consists of several layers, each of which performs a specific function.
- Mesh zone.
- Bundle zone.
- Zona glomerulosa.
Androgens, sex hormones, are synthesized in the reticular zone. They are responsible for the development of secondary sexual characteristics and influence:
- state of libido - sexual desire in men and women;
- blood cholesterol and lipid levels;
- fat deposition;
- increase in muscle strength and mass.
Regardless of gender, each body produces both male and female sex hormones. The difference is in their quantity. For example, women synthesize estrogen and progesterone, which are responsible for reproductive function - conception and birth of children, but also testosterone in a small amount, which is considered the male hormone.
RADIAL ANATOMY OF THE ADRENAL GLANDS
Human radiation anatomy. Ed. Trofimova T.N. 2005 year
- Normal anatomy and topography of the adrenal glands
- Ultrasound anatomy of the adrenal glands
- CT anatomy of the adrenal glands
- MRI anatomy of the adrenal glands
NORMAL ANATOMY AND TOPOGRAPHY OF THE ADRENAL GLANDS
The adrenal gland (glandulae suprarenales) is a paired endocrine organ located in the retroperitoneal space above the upper poles of the kidneys.
The adrenal glands consist of two morphofunctionally independent endocrine glands - the medulla and the cortex, which have different embryonic origins [1,4].
The structure of the adrenal glands changes with age. In newborns (children in the first two weeks of life), the adrenal glands are large; their height can range from 23 mm to 55 mm and exceed the vertical size of the kidney. The width of the adrenal gland is 23-25 mm, the thickness immediately after birth can reach 10 mm. By the end of the third year of life, the adrenal glands are minimal in size. Restoration of the mass of the gland to that of a newborn occurs by 5 years.
The most active growth of the adrenal glands is observed in the prepubertal and pubertal periods, mainly due to an increase in the volume of the cortical layer. At this time, differentiation of cells of the adrenal cortex occurs with the delimitation of zones characteristic of an adult. In older children, the height of the adrenal gland can reach 25-30 mm, and the thickness does not exceed 8-10 mm. After 40 years, a gradual thinning of the zona reticularis, which produces sex hormones, occurs, and in the menopause, almost the entire adrenal cortex is occupied by the zona fasciculata [4].
The shape of the adrenal gland resembles a cone flattened in the anteroposterior direction with a smoothed apex, which has three surfaces: anterior, posterior and inferior (renal). The adrenal hilum is located on the anterior surface. The height of the adrenal glands of an adult is from 25 to 45 mm, width - from 20 to 35 mm, thickness usually does not exceed 10 mm [5-8].
The adrenal glands are located in the retroperitoneal space at the level of Thx|X|1, above the upper poles of the kidneys. Posteriorly and superiorly they are adjacent to the lumbar part of the diaphragm. The aorta is located medial to the left adrenal gland. In front and above it is the tail of the pancreas with the vessels of the spleen lying along it, as well as the cardiac part of the stomach. The inferior vena cava is adjacent to the right adrenal gland in front and on the medial side. The anterior sections of the gland on the right are adjacent to the superior flexure of the duodenum. The lateral portion of the right adrenal gland is in contact with the visceral surface of the liver. Part of the anterior surface of the left adrenal gland is covered by the parietal peritoneum. Together with the kidneys, the adrenal glands are enclosed in the fatty capsule of the kidney.
ULTRASONIC ANATOMY OF THE ADRENAL GLANDS
The adrenal glands are located above the upper pole of the kidneys and are slightly displaced anteriorly and medially, and have a homogeneous fine-grained echostructure. Their echogenicity exceeds that of the liver, spleen and renal parenchyma, but is slightly lower than the echogenicity of the middle structures of the kidney. The shape of the adrenal glands can be varied and, during ultrasound examination, is compared with a cone, trapezoid, truncated pyramid, sickle, “chef’s hat”, but is always associated with one or another type of triangle.
The size of the adrenal glands is variable. Ultrasound examination evaluates three dimensions of the adrenal gland: width, height and thickness. The width is taken to be the base of the “triangle” of the gland adjacent to the kidney in the frontal plane. The vertical dimension (height) is measured along a line perpendicular to the base. Thickness, or anteroposterior dimension, is measured at the base of the gland in the sagittal plane. On average, the height of the adult adrenal gland during ultrasound examination is 18-25 mm, width - 16-23 mm, thickness - 12-14 mm.
The criterion for the correct examination of the right adrenal gland is to obtain on a longitudinal scanogram a triangle formed by the inferior vena cava, the upper pole of the right kidney and the inferoposterior edge of the liver (Fig. 12.1).
The criterion for the accuracy of the study of the left adrenal gland is to obtain a triangle formed by the aorta, the upper pole of the left kidney and the spleen on a longitudinal scanogram (Fig. 12.2).
Rice. 12.1. Ultrasound of the right adrenal gland, longitudinal scanning. a – diagram; b — echogram.
1 – liver; 2 - right kidney; 3 - right adrenal gland; 4 - inferior vena cava.
Rice. 12.2. Ultrasound of the left adrenal gland, longitudinal scanning. a - diagram; b — echogram.
1 - spleen; 2 - upper pole of the left kidney; 3 - left adrenal gland; 4 - aorta.
Rice. 12.3. Ultrasound of the adrenal gland of a newborn.
1 - adrenal gland; 2 - right kidney; 3 - liver.
During ultrasound scanning, the adrenal glands of a newborn have their own characteristics, which are associated with the presence of a developed fetal cortical zone. The glands are distinguished by their large size, reduced echogenicity, homogeneous echostructure and are well differentiated against the background of hyper-echoic fatty tissue (Fig. 12.3) [2].
CT ANATOMY OF THE ADRENAL GLANDS
The adrenal gland area must be examined using thin sections (3 mm or 5 mm thick). Using a series of computed tomograms, an image of the adrenal glands is obtained in the axial scanning plane.
Right adrenal gland. Located 10-20 mm anterior to the upper pole of the right kidney. Localized posterior to the inferior vena cava. On the lateral side, the gland is delimited from the visceral surface of the right lobe of the liver by retroperitoneal fatty tissue, which, depending on the characteristics of the patient’s constitution, has a different volume. The medial surface of the adrenal gland is parallel to the right crus of the diaphragm. The lower parts of the gland are located above the vascular pedicle of the kidney. The right adrenal gland is located approximately 10 mm lateral to the vertebral body and 5-10 mm posterior to the anterior edge of the vertebral body (Fig. 12.4).
Left adrenal gland. The medial parts of the gland are located outward from the left leg of the diaphragm, but, unlike the right adrenal gland, are not parallel to it. The left adrenal gland is located anterior to the outer edge of the vertebral body and the upper pole of the left kidney. The aorta is localized medial to the gland. The lower parts of the left adrenal gland do not reach the vascular pedicle of the kidney. The tail of the pancreas is located anterior and/or slightly lateral to the left adrenal gland and is visualized together with it in 95% of patients, so it is a good landmark when locating the adrenal gland. The vessels of the spleen are located posterior to the lateral parts of the gland. In some cases, the adrenal gland may come into contact with the body of the stomach (Fig. 12.5).
In 51% of cases, the right adrenal gland is located above the left, in 34% of cases, the right and left adrenal glands are at the same level, in 15% of cases, the gland on the left side is localized more cranially than the right.
The adrenal glands are divided into a body, lateral and medial crura.
The shape of the glands is variable. The right adrenal gland often has a linear shape, parallel to the right leg of the diaphragm. Its lateral leg is usually shorter than the medial one. The right adrenal gland may be a horizontal linear structure or have a K-shaped configuration. The left adrenal gland often has an inverted V- and Y-shape. There are linear, triangular and crescent-shaped configurations of the gland. If the legs of the left adrenal gland are unequal in length, then the size of the lateral leg prevails. The shape of the adrenal gland on CT scans largely depends on the level of scanning. The contours of a normal gland are clear and even.
The size of the adrenal glands is usually measured using the method developed by J. Montagne et al. The height, width and thickness of the gland are determined. The height of the adrenal gland is calculated by the sum of the sections on which it was imaged. The width of the adrenal gland coincides with the largest ventrodorsal size of the gland (the distance between its anterior and posterior sections).
Rice. 12.4. CT scan of the abdomen at the level of the adrenal glands.
1 – liver; 2 - right adrenaline; 3 - aorta; 4 spleen.
Rice. 12.5. CT scan of the abdomen at the level of the adrenal glands.
1 - left adrenal gland; 2 - spleen; 3 - body of the pancreas; 4 - aorta.
The thickness of the organ is taken to be the largest size when measured perpendicular to the long axis of the gland or one of its legs. Thickness is usually measured in the projection of the body of the adrenal gland, at the junction of its lateral and medial legs.
The height of the adrenal gland in 92% of cases on the right and in 96% of cases on the left ranges from 20 to 35 mm. The width of the adrenal gland varies from 20-25 mm, but in some cases the normal adrenal gland can be up to 30 mm wide. The thickness of the organ should not exceed 10 mm.
The density of the adrenal glands is 15-30 HU. After administration of a contrast agent, densitometric indicators increase by 10-15 HU.
MRI ANATOMY OF THE ADRENAL GLAND
The location, size and shape of the adrenal glands in the MRI image do not differ from the indicated parameters in CT. A study in the coronal plane makes it possible to more clearly assess the location of the gland and its height (Fig. 12.6).
MRI allows visualization of the right adrenal gland in 99% of adult patients, the left adrenal gland in 91% of subjects.
Normal adrenal glands on T1- and T2-weighted images are homogeneous, hypointense structures that are well differentiated against the background of surrounding fatty tissue (Fig. 12.7). When using T2-V I, the signal from the adrenal parenchyma is comparable to that from the paravertebral muscles; slightly exceeds the signal from the liver and renal cortex and significantly exceeds the signal from the legs of the diaphragm (Fig. 12.8).
The use of STIR PI makes it possible to suppress the signal from retroperitoneal fat and clearly visualize the adrenal glands, which have a moderately hyperintense signal. None of the pulse sequences makes it possible to distinguish between the cortex and medulla of the gland. After administration of a contrast agent, its accumulation occurs within 2-5 minutes. The adrenal glands are bright, hyperintense structures. “Washing out” of the contrast agent occurs quickly - within 5 minutes.
The adrenal glands of a newborn have characteristic CT and MRI signs (in the first two weeks of postnatal development). They are distinguished by their large size, due to the significant volume of the embryonic fetal zone. The cells of the fetal zone are poor in lipids, therefore, on T2-WI and STIR IP, the signal from the adrenal glands exceeds this indicator in an adult.
Rice. 12.6. MRI of the adrenal glands. T1-weighted image, coronal plane.
1 - right adrenal gland; 2 - left adrenal gland; 3 - right kidney; 4 - left kidney.
Rice. 12.7. MRI of the adrenal glands. T1-weighted image, axial plane.
1 - left adrenal gland; 2 - right adrenal gland.
Rice. 12.8. MRI of the adrenal glands. T2-weighted image, axial plane.
1 - left adrenal gland; 2 - right adrenal gland.
Beam zone
This zone is responsible for the synthesis of glucocorticosteroids. They are responsible for protein, carbohydrate and fat metabolism in the body. Closely related to the production of insulin and catecholamines.
Glucocorticosteroids reduce the immune response, inhibit inflammatory processes, gradually suppressing them.
One of the hormones in the zona fasciculata is cortisol. It takes part in carbohydrate metabolism and preserves the body's energy resources. The level of cortisol in the body is constantly changing - there is more of it in the morning than in the evening or at night.
Zona glomerulosa
The zona glomerulosa of the adrenal cortex is responsible for mineral metabolism in the body. The hormones produced here normalize the functioning of the renal tubules, so excess fluid leaves the body, which, in turn, keeps blood pressure levels normal.
The following hormones are secreted in the zona glomerulosa:
- Aldosterone. Its function is to maintain the balance of sodium and potassium ions in the blood. The hormone takes part in water-salt metabolism, improves blood circulation, and increases blood pressure.
- Corticosterone is a hormone with low activity and is involved in mineral balance.
- Deoxycorticosterone also regulates water-salt balance, gives strength to the skeleton and muscle tissue.
Our clinics in St. Petersburg
Medical center on Pionerskaya Polikarpov Alley 6k2 Primorsky district
- Pionerskaya
- Specific
- Commandant's
Medical center South-West Marshal Zhukov Ave. 28k2 Kirovsky district
- Avtovo
- Avenue of Veterans
- Leninsky Prospekt
Medical center in Devyatkino Okhtinskaya alley 18 Vsevolozhsk district
- Devyatkino
- Civil Prospect
- Academic
For detailed information and to make an appointment, you can call +7 (812) 640-55-25
Make an appointment
The adrenal glands consist of an inner medulla and cortex. Aldosterone is produced in the upper part of the cortex, which is the zona glomerulosa. The inner layer produces the synthesis of sex hormones. The medulla produces norepinephrine and adrenaline. The main glucocorticoid is cortisol. They regulate metabolism and have an anti-inflammatory effect. Aldosterone is the most active mineralocorticoid. It increases the excretion of potassium and reduces the excretion of sodium from the body.
Functions of the adrenal glands in the body:
- protective-adaptive;
- control of metabolic processes;
- production of hormones, including sex hormones.
How do adrenal gland diseases manifest?
When the balance of hormones in the body is disturbed, its functioning occurs. Symptoms appear according to a dependence pattern, that is, a specific substance produced in the adrenal glands is responsible for a symptom unique to it. For example, with aldosterone deficiency, sodium is excreted from the body along with the urine, which lowers blood pressure and significantly increases the potassium content in the blood.
To avoid serious consequences, you need to consult a doctor at the first signs of adrenal disease. These include:
- muscle weakness;
- constant fatigue;
- severe weight loss is a consequence of decreased appetite;
- low stress resistance;
- high irritability;
- rapid and constant fatigue;
- sleep problems;
- dizziness, headaches.
Next, the most common diseases of the adrenal glands, which have their own symptoms and causes, require specific treatment tactics.
Hyperfunction of the adrenal medulla
113114115116117118119>
The function of the medulla is enhanced, as a rule, when the body finds itself in extreme conditions, the action of nociceptive (from the Latin
- harm) irritants. Under these conditions, the sympathoadrenal system is activated, which is part of the general adaptive syndrome. Sometimes the basis of hyperfunction is the formation of a tumor from cells of the adrenal medulla or extra-adrenal chromaffin tissue - chromaffinoma. It is more often benign (pheochromocytoma) and less often malignant (pheochromoblastoma). The tumor is relatively rare - according to autopsies, in 0.04%. However, among patients with arterial hypertension it is much more common. The size of the tumor varies widely - from microscopic to tumors weighing 3.5 kg. Chromaffin cells secrete catecholamines - adrenaline, norepinephrine, a precursor - dopamine and sometimes serotonin. The amount and ratio of secreted products vary dramatically, which creates large differences in the clinical manifestations of the disease.
Cardiovascular syndrome is manifested primarily by a paroxysmal or constant increase in blood pressure. Various changes in heart activity are observed: tachycardia or bradycardia, rhythm disturbances such as extrasystole, His bundle block, atrial fibrillation.
Metabolic disorders are characterized by symptoms of moderate diabetes, thyrotoxicosis, and hypercholesterolemia. For patients with pheochromocytoma, early development of atherosclerosis is typical.
Neuropsychiatric syndrome manifests itself during paroxysms with dizziness, headache, hallucinations, increased excitability of the nervous system, and convulsions.
Less commonly, pheochromocytoma is accompanied by gastrointestinal syndrome. It is expressed in nausea, vomiting, constipation, and sometimes in ulceration of the intestinal wall of the stomach with subsequent development of bleeding.
On the contrary, hypofunction of the adrenal medulla can serve as one of the pathogenetic factors of hypotonic conditions.
Pathophysiology of the thyroid gland
Hyperthyroidism
Hyperthyroidism is a syndrome caused by increased thyroid function. Severe hyperthyroidism is called thyrotoxicosis. Hyperthyroidism, depending on the location where the primary disorder occurs, can be divided into primary, secondary and tertiary. The causes of primary hyperthyroidism may be dysfunction of the thyroid gland, which develops in diseases such as diffuse toxic goiter (Graves' disease, Graves' disease, Parry's disease), thyrotoxic adenoma of the thyroid gland. The cause of secondary hyperthyroidism may be the development of a TSH-secreting tumor of the adenohypophysis, and the cause of tertiary hyperthyroidism may be a disorder in the hypothalamus.
In general, the most common cause of hyperthyroidism is diffuse toxic goiter. It is believed that with this disease, the body produces thyroid-stimulating antibodies, which, like TSH, are able to bind to receptors on the basement membrane of the thyrocyte, which leads to cell activation. At the same time, the level of TSH in the blood of patients is reduced by a feedback mechanism.
Hyperthyroidism is accompanied by impaired energy and increased basal metabolism, increased oxygen consumption, disorders of various types of metabolism, weight loss, dysfunction of the central nervous system, cardiovascular system and other organs.
Energy exchange. Triiodothyronine uncouples oxidation and phosphorylation in cell mitochondria, as a result of which the energy of oxidation of NAD2H and NADP2H is not accumulated in ATP and is dissipated. A decrease in ATP synthesis increases the concentration of its precursors - ADP and inorganic phosphate; the transfer of ADP into mitochondria also changes, since triiodothyronine binds to the ADP transporter translocase, which, in turn, enhances oxidative processes and thereby energy dissipation. This leads to an increase in basal metabolism.
Carbohydrate metabolism. With hyperthyroidism, carbohydrate metabolism increases and glucose utilization by tissues increases. Phosphorylase of the liver and muscles is activated, resulting in increased glycogenolysis and depletion of these tissues in glycogen. Hexokinase activity and glucose absorption in the intestine increase, which is accompanied by nutritional hyperglycemia. In-
liver sulinase. This, together with hyperglycemia, causes strained functioning of the insular apparatus and, if it is defective, can lead to the development of diabetes mellitus. In addition, strengthening of the pentose phosphate pathway of carbohydrate metabolism promotes increased formation of NADP2H.
Protein metabolism. Thyroid hormones in large doses have a mainly catabolic effect on protein metabolism, which leads to a negative nitrogen balance. The excretion of nitrogen, phosphorus and potassium in the urine increases, indicating cellular breakdown. The release of ammonia increases. The level of residual nitrogen and amino acid nitrogen in the blood increases. Increased protein catabolism is associated with the development of symptoms of diffuse toxic goiter such as muscle atrophy and osteoporosis.
Fat metabolism. Due to increased energy metabolism, patients with thyrotoxicosis lose weight mainly due to a decrease in fat reserves in fat depots. A decrease in fat reserves occurs due to: a) mobilization of fats from the depot due to sensitization of sympathetic nerve endings in adipose tissue; b) acceleration of fat oxidation in the liver; c) inhibition of the transition of carbohydrates to fats. Due to increased fat oxidation, the formation of ketone bodies increases. With a simultaneous deficiency of carbohydrates, this leads to a violation of their oxidation and, consequently, to hyperketonemia and ketonuria. Increased breakdown of fat leads to the development of general weight loss in patients with diffuse toxic goiter.
Water and mineral metabolism. An increase in the concentration of thyroid hormones in the blood causes an increase in: a) the relative content of water in the body due to weight loss; b) plasma volume; c) the rate of water filtration through capillary walls; d) diuresis due to increased renal blood flow and glomerular filtration; e) sweating; f) loss of water with exhaled air. At the same time, the excretion of calcium, phosphorus and potassium from the body is enhanced.
Central nervous system and other organs. Thyroid hormones have a pronounced effect on the central nervous system. The excitability of the cerebral cortex increases. Toxic-degenerative changes develop in the cells of the cortex, brain stem and anterior horns of the spinal cord. The excitability of the hypothalamic autonomic centers changes, and in connection with this, the function of the internal organs.
On the part of the cardiovascular system, persistent tachycardia and a tendency to atrial fibrillation are noted. This phenomenon is based on an increase in the sensitivity of the myocardium to adrenaline and norepinephrine due to an increase in the number of beta-adrenergic receptors under the influence of thyroid hormones. It is also possible that the breakdown of thyroid hormones produces active products that can function as pseudocatecholamines. Increased work of the heart causes its hypertrophy and dystrophic changes. An increase in excitation of the sympathetic nervous system leads to an increase in arteriolar tone and the development of hypertension and tremor. A decrease in the amount of glycogen in the liver reduces its detoxification function and ability to synthesize proteins. Increased skin humidity and temperature. Exophthalmos (bulging eyes) that develops in some cases with diffuse toxic goiter (Fig. 20-15), as well as changes in the skin of the legs and hands (acropathy), may be a consequence of autoimmune tissue damage.
Rice. 20-15. Thyrotoxicosis in a 33-year-old woman (according to N.A. Shereshevsky)
Hypothyroidism
Hypothyroidism is a condition that occurs when there is a lack of thyroid hormones in the body. So
Like hyperthyroidism, it can be primary, secondary and tertiary. Primary hypothyroidism occurs with Hashimoto's thyroiditis, defects in the biosynthesis of thyroid hormones, thyroidectomy, treatment with radioactive iodine, insufficient intake of iodine in the body and other pathological processes in the gland. Secondary and tertiary hypothyroidism are a consequence of loss of regulatory influences (damage to the pituitary gland, deficiency of thyrotropin-releasing hormone). The most severe form of hypothyroidism in adults
called myxedema. The syndrome that develops in children due to complete insufficiency of the thyroid gland is called cretinism. Cretinism is characterized by severe growth retardation and a peculiar appearance of the patient (Fig. 20-16). Cretinism is usually based on aplasia of the thyroid gland.
Thyroidectomy in the experiment is accompanied by retardation in the growth of young animals, delayed growth of tubular bones and sexual development. There are deviations from the norm in appearance. The configuration of the skull changes - the front part of the face shortens, and the back part takes on a spherical shape, and the development of teeth stops. In dogs, the limbs become thick, movements become clumsy, and hair growth stops. Mucoid swelling of the subcutaneous tissue develops due to retention of water, sodium chloride and accumulation of mucous membranes in the connective tissue.
lysaccharides with hydrophilic properties. If well maintained, animals can live for months and years.
With hypothyroidism, the following metabolic and organ function disorders are observed:
Energy exchange. Hypothyroidism is accompanied by a decrease in the intensity of oxidative processes, which leads to a decrease in basal metabolism.
Protein metabolism. With functional insufficiency of the thyroid gland, the intensity of protein synthesis decreases. Evidence of this is the inhibition of the rate of incorporation of methionine into tissue proteins. At the same time, the catabolism of amino acids increases and the RNA content in tissues decreases.
Carbohydrate metabolism. The intensity of carbohydrate metabolism decreases. The glycogen content in the liver increases due to a decrease in phosphorylase activity. As a result of weakening hexokinase activity, glucose absorption in the intestine decreases. A consequence of slowing down oxidative processes in tissues may be the development of hyperketonemia.
Fat metabolism. The rate of cholesterol synthesis in the liver and adrenal glands decreases, but its breakdown slows down even more, which leads to hypercholesterolemia and contributes to the development of atherosclerosis.
After thyroidectomy in dogs, the excitability of the central nervous system decreases. People with hypothyroidism experience slowed mental reactions, weakened memory, and in severe cases, dementia.
Endemic goiter. A special form of hypothyroidism is endemic goiter. It develops in certain geographic areas where the population does not get enough iodine from their diet. Lack of iodine reduces the synthesis of thyroid hormones, which, through a feedback mechanism, increases the secretion of TSH by the pituitary gland. This causes hyperplasia of the gland, which initially compensates for the lack of thyroid hormones. However, with continued iodine deficiency, this compensation is insufficient for the formation of thyroid hormones, and hypothyroidism develops, which in advanced cases can develop into myxedema and cretinism (Fig. 20-17). Prophylactic administration of iodine prevents the occurrence of this disease. For this purpose, 0.002% sodium or potassium iodide is added to table salt. Consuming 6 g of salt daily means taking in 120 mcg of iodide, which is the optimal daily dose for adults.
Rice. 20-17. A group of endemic cretins from the town of Aarau (Germany, 1908) (after W. Falta, 1913)
113114115116117118119>
Date added: 2016-07-11; views: 4201; ORDER A WORK WRITING
Find out more:
Addison's disease
Other names for Addison's disease are primary adrenal insufficiency and hypocortisolism.
A rare pathology, accounting for 50–100 cases per million per year. It is diagnosed in both women and men. Average age is from 20 to 40 years.
The disease affects all three zones of the adrenal cortex. Characterized by a deficiency in the production of corticosteroids. Violation of hormone synthesis causes serious complications in the body.
The cause of destruction of the adrenal cortex is pathogenic microorganisms that have entered the body - viruses, bacteria, fungi, as well as immune disorders.
Manifestations of Addison's disease:
- decreased blood pressure;
- fatigue, weakness, lack of physical strength;
- lack of appetite;
- disruption of the digestive system;
- pigmentation of the skin, the appearance of dark spots on the mucous membranes;
- chills;
- increase in body temperature.
To identify the disease, it is necessary to take tests to measure the level of cortisol in the blood. The condition of the adrenal cortex and the functioning of the glands are examined.
Treatment is taking medications containing corticosteroids throughout life. It is possible to administer hydrocortisone intramuscularly.
Itsenko-Cushing's disease
Refers to neuroendocrine pathologies. The reason is a malfunction of the hypothalamic-pituitary system as a result of brain injury or infections suffered by a person. Characterized by the production of excessive amounts of corticosteroids by the adrenal glands.
Itsenko-Cushing's disease is a rare pathology. It is diagnosed mainly in women aged 30 to 45 years.
A malfunction in the hypothalamic-pituitary system provokes disturbances in the body. The connection between the adrenal glands, hypothalamus and pituitary gland is lost. Signals sent to the hypothalamus provoke excess production of hormones that release adrenocorticotropic hormone (ACTH) in the pituitary gland, which stimulates the release of the substance into the blood. Excessive amounts of ACTH affect the adrenal glands, which begin to increase the production of a lot of corticosteroids.
As the pathology progresses, there is a visible increase in size of the pituitary gland and adrenal glands.
Signs of Itsenko-Cushing's disease:
- headaches, migraines;
- progressive hypertension;
- amyotrophy;
- formation of a moon-shaped face;
- lack of menstruation in women;
- development of osteoporosis;
- facial hair growth in women.
To identify the disease, blood and urine tests are prescribed, which determine excess amounts of ACTH and cortisol. Additionally, an instrumental examination is carried out.
The goal of therapy is to restore impaired metabolism, normalize the functioning of the hypothalamus, and normalize the synthesis of corticosteroids.
Pheochromocytoma
Pheochromocytoma is a tumor that develops in the adrenal medulla. It is based on chromaffin bodies, which contribute to the synthesis of large amounts of catecholamines.
The cause is arterial hypertension and catecholamine crises.
Symptoms of pheochromocytoma:
- high blood pressure, hypertensive crises;
- headache;
- increased sweating;
- dyspnea;
- dehydration of the body;
- convulsions;
- cardiomyopathy;
- high blood sugar.
To diagnose the disease, tests are prescribed to determine catecholamine metabolites in the urine and blood. To identify the formation, hardware methods are used - MRI and ultrasound scanning.
Treatment is medicinal, aimed at reducing the severity of paroxysmal attacks.
Hyperaldosteronism
Another name for the pathology is Kohn syndrome. A condition in which the adrenal cortex produces excessive amounts of aldosterone and deoxycorticosterone.
The causes of hyperaldosteronism are a malignant formation in the adrenal cortex or hyperplasia of cortical tissue.
Kohn syndrome has primary and secondary degrees of development. Symptoms of the primary degree of the disease:
- increased blood pressure;
- headache;
- heart rhythm disturbance;
- cardialgia – pain in the left side of the chest;
- decreased visual acuity.
Secondary symptoms result from excess potassium and sodium deficiency:
- swelling;
- chronic renal failure;
- fundus deformation;
- arterial hypertension.
Diagnostic measures include determining the level of potassium and sodium, aldosterone in the blood and urine.
Therapy depends on the results of the examination. More often this is drug treatment with the prescription of hormonal drugs. If a tumor is present, surgery may be performed.
Symptoms of adrenal diseases
The manifestations of adrenal gland diseases depend on which hormone or hormones are produced in insufficient or excessive quantities. For example, an excess of mineralocorticoids causes an increase in blood pressure and a decrease in potassium levels, an excess of sex hormones causes impaired sexual development, excessive growth of facial hair, etc. A life-threatening condition is acute adrenal insufficiency, which occurs when there is a sharp decrease or cessation of hormone production by the adrenal cortex.
Hyperplasia of the adrenal cortex
This is an increase in the size of the adrenal cortex, which leads to increased synthesis of androgens. The main reason is genetic, that is, the disease is congenital.
There are three main forms of hyperplasia:
- Simple virilizing. There is an increase in the synthesis of androgens, as a result of which the genitals and muscle tissue become enlarged, and hair grows rapidly all over the body, regardless of the gender of the sick person.
- With salt loss syndrome. Characterized by excess potassium and deficiency of other hormones.
- Hypertensive. Excessive production of androgens and corticosteroids occurs, as a result of which arterial hypertension develops and visual function deteriorates.
Symptoms of adrenal hyperplasia:
- early hair growth on the body and genitals;
- growth is less than normal;
- rough voice;
- memory problems;
- frequent psychoses;
- weakness of the muscular system.
Signs appear in childhood. To clarify the diagnosis, tests and laboratory tests are prescribed.
Treatment is medicinal and includes constant use of hormonal drugs. In severe cases of the disease, surgical intervention is indicated.
Hyperfunctional states of the adrenal cortex
Increased secretion of male sex hormones (androgens) in women leads to a condition called “hyperandrogenism” (symptoms of the disease include excessive body hair, miscarriage, menstrual irregularities, lack of ovulation, infertility, obesity, deepening of the voice, acne on the skin).
Excess male hormones in women affect her ability to conceive and bear children. A pregnant woman with hyperandrogenism should be under the strict supervision of a local antenatal clinic doctor due to the possible threat of miscarriage. It is necessary to periodically monitor the level of male sex hormones in her body in order to adjust the prescribed treatment regimen.
As a rule, hyperandrogenism is treated with synthetic cortisol analogues (prednisone, dexamethasone). These drugs are prescribed to suppress the influence of the pituitary gland on the adrenal glands.
The occurrence of adrenogenital syndrome is associated with deficiency of C21-hydroxylase. Currently, the term “dysfunction” or “hyperfunction of the adrenal glands” is more often used in medical practice.
| Adrenogenital syndrome (hyperandrogenism) | |
| Form of adrenal dysfunction | Manifestations |
| Classic shape | In the classic form of adrenogenital syndrome, increased secretion of androgens begins in utero, at 9-10 weeks after gestation. An excess of male sex hormones disrupts the formation of the external genital organs of the future girl (the internal genital organs of a female fetus already have a structure characteristic of the female sex by the 9-10th week of intrauterine development). The second manifestation of congenital hyperandrogenism is adrenal hyperplasia. After birth, such a child should be registered with a pediatric endocrinologist. |
| Pubertal form of congenital adrenal dysfunction | Adrenogenital syndrome begins to manifest itself in a child during puberty, when the hormonal function of the adrenal cortex begins to increase. As a rule, the disease begins to manifest itself in girls at 12-13 years of age. Characteristic manifestations of the pubertal form of adrenogenital syndrome:
|
| Postpubertal form | It is usually detected at the age of 25-29, usually in connection with an unsuccessful pregnancy (miscarriage, medical abortion, non-developing pregnancy). Patients complain of irregularities in the menstrual cycle - a tendency to delay menstruation and a general lengthening of the cycle. Due to the late development of hyperandrogenism, its manifestations are blurred: excess hair growth is poorly expressed, the mammary glands have normal development, the figure of a young woman is developed according to the female type. |
With the secretion of increased doses of adrenal hormones, certain clinical manifestations occur. Excessive release of cortisol usually leads to Cushing's syndrome, and "excess" production of male sex hormones in women leads to masculinization. Normally, cortisol inhibits the production of adrenocorticotropin (ACTH), secreted by the pituitary gland. Chronic cortisol deficiency is manifested by adrenogenital syndrome (the number of male sex hormones in the blood increases sharply).
Adrenal insufficiency
This is a dysfunction of the adrenal glands of an autoimmune nature. There are two types - acute and chronic.
Chronic insufficiency of the adrenal cortex occurs as a result of destructive changes in the glandular tissues of the organ. The main causes are previous infectious diseases, a pituitary tumor or macroadenoma, necrosis or inhibition of the anterior lobe of the pituitary gland.
The acute form develops against the background of the chronic form. It almost never occurs on its own, with the exception of sepsis or sudden hemorrhage in the adrenal glands.
Symptoms of adrenal insufficiency:
- weakness, constant loss of strength;
- loss of appetite, resulting in a sharp decrease in body weight;
- hyperpigmentation of the skin;
- arterial hypotension;
- decreased blood sugar levels;
- frequent urination;
- nausea turning into vomiting;
- uncharacteristic stool.
Adrenal dysfunction
Hypoadrenia
Stress
- this is the tension of the body associated with an unfavorable effect on it or the emergence of a situation that threatens the life or well-being of the individual. Simply put, stress is a protective reaction against dangerous influences. A stressor (factor that causes stress) can be both physiological and psychological. The human body reacts to stress in the way that has been inherent in it since ancient times - by mobilizing its strength and going into full combat readiness mode. In response to danger, our adrenal glands begin to produce increased amounts of hormones.
So how does our body respond and adapt to stress?
Stress activates a powerful release of cortisol and adrenaline into the blood. As a result, blood pressure increases, heart rate increases, and muscle tone increases. There is nothing wrong with short-term stress on all the body’s resources; it is much worse if stress “becomes a habit” and becomes chronic. Constant nervous overload leads to depletion of adrenal function - hypoadrenia.
The second cause of hypoadrenia may be pregnancy
.
Pregnancy is a big burden on the adrenal glands of both the mother and the growing fetus. Typically, the fetal adrenal glands begin to work only by the sixth month of pregnancy (the beginning of the third trimester). If a pregnant woman is in a state of hypoadrenia (insufficient adrenal function), then the fetal adrenal glands begin to work “for two”, supporting her.
Therefore, many women in the final trimester feel a surge of strength compared to the previous weeks of pregnancy. However, the stress that the adrenal glands experience can have a direct impact on the health of the newborn baby: he may show signs of hypoadrenia. This subsequently affects the baby’s health:
- the capabilities of the immune system are reduced (frequently ill child)
- allergic mood of the body
The mother, finding herself without the support of the fetal adrenal glands, returns to a state of hypoadrenia after the birth of the child, as a result of which both mother and child have to be treated.
Tyrical pathologies of the adrenal glands are divided into hyperfunctional
and
hypofunctional
.
Inflammation of the adrenal glands
Inflammatory processes in the adrenal glands most often occur against the background of tuberculous damage to their cortex. The peculiarity of the pathology is its slow development.
Characteristic symptoms of inflammation:
- feeling of constant fatigue;
- low stress resistance;
- regular nagging headaches;
- bad breath;
- nausea turning into vomiting.
With an advanced form, there is a high probability of developing a chronic inflammatory process.
Adrenal tuberculosis
Adrenal tuberculosis is a rare pathology. It is characterized by the accumulation of calcifications in the tissues of the adrenal glands.
More often diagnosed in childhood and adolescence. The reason is the penetration of the tuberculosis bacillus from the infected lungs into the adrenal glands through the general bloodstream.
Symptoms of adrenal tuberculosis:
- low blood pressure;
- disruption of the gastrointestinal tract, expressed by diarrhea, nausea with vomiting attacks;
- hypoglycemia;
- constant weakness, feeling tired;
- myocardial dystrophy.
Cyst
This is a benign formation in the tissues of the adrenal glands. The tumor appears rarely and becomes dangerous only when it degenerates into malignant. A dangerous condition is when an adrenal cyst ruptures.
Tumor symptoms:
- pain in the lower back, back, sides;
- impaired renal function;
- an increase in the size of the adrenal glands;
- increased blood pressure as a result of compression of the renal artery.
Diagnosis of an adrenal cyst is difficult due to its small size. Pathology can only be detected when the tumor progresses and grows.
Dangerous tumors
If the cyst is a benign formation, then there are a number of tumors prone to malignancy.
The most common of them:
- aldosteroma;
- andosteroma;
- glucocorticosteroma;
- corticoestroma.
The exact reasons for the development of formations are difficult to establish, but there is a high probability of their hormonal activity against the background of the following provoking factors:
- cell hyperplasia and proliferation of adrenal tissue;
- excessive amounts of hormones produced;
- thyroid cancer;
- diseases associated with congenital pathologies of the skin, eye membrane, and cerebral vessels.
The localization of tumors varies. They can form in both the medulla and cortical layers. Characteristic symptoms:
- high blood pressure;
- delay in the process of sexual development;
- frequent nausea, turning into vomiting;
- pain in the abdomen, chest area;
- change in skin color on the face - turns pale, red, acquires a bluish tint;
- a sharp change in blood glucose levels;
- dry mouth;
- convulsions;
- tremor of the limbs;
- increased irritability, excitability;
- increased anxiety, constant feeling of fear.
Hypofunctional conditions of the adrenal cortex
Chronic adrenal insufficiency is called Addison's disease. This disease is characterized by the fact that the adrenal glands lose the ability to produce hormones (especially cortisol) in sufficient quantities.
The main symptoms of Addison's disease:
- Weakness, fatigue for a long time
- Loss of muscle mass
- Decreased appetite
- Nausea, vomiting, diarrhea, difficulty swallowing, nagging abdominal pain
- Hypotension (low blood pressure)
- Appearance of spots and hyperpigmentation on the skin
- Depression, short temper, aggressiveness, irritability, internal tension, nervousness, anxiety, tearfulness
- Change in taste preferences, addiction to salty foods
- Decreased blood glucose levels
- Disappearance of menstruation in women
- Changes in blood composition (increased number of eosinophils)
- Convulsions, paresthesia, tremors of hands and head
- Increased urine output
- Dehydration (dehydration) of the body
- Heart problems (tachycardia)
An acute condition that can threaten the patient’s life is “Addisonian crisis.” The disease is treated with synthetic corticosteroids (the dose of the drug is established after examining the patient).