The structure of the kidney vessels
The renal arteries arise from the abdominal aorta just below the superior mesenteric artery - at the level of the second lumbar vertebra.
Anterior to the renal artery is the renal vein. At the hilum of the kidney, both vessels are located anterior to the pelvis. The RCA passes behind the inferior vena cava. The LPV passes through the “tweezers” between the aorta and the superior mesenteric artery. Sometimes a ring-shaped left vein is found, in which case one branch is located in front and the other behind the aorta.
Click on pictures to enlarge.
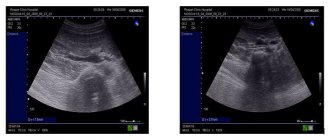
To study the vessels of the kidney, a 2.5-7 MHz convex sensor is used. The patient is positioned supine, the sensor is placed in the epigastrium. Assess the aorta from the celiac trunk to the bifurcation in B-mode and color flow. Trace the course of the RAA and LPA from the aorta to the renal hilum.
Drawing. In the CD mode, on the longitudinal (1) and transverse (2) sections, the RAA and LPA extend from the aorta. The vessels are directed to the gates of the kidney. Anterior to the renal artery is the renal vein (3).
Drawing. The renal veins drain into the inferior vena cava (1, 2). The aortomesenteric “tweezers” may compress the left ventricular vein (3).
Drawing. At the hilum of the kidney, the main renal artery is divided into five segmental ones: posterior, apical, superior, middle and inferior. The segmental arteries are divided into interlobar arteries, which are located between the pyramids of the kidney. The interlobar arteries continue into the arcuate → interlobular → glomerular afferent arterioles → capillary glomeruli. Blood from the glomerulus drains through the efferent arteriole into the interlobular veins. The interlobular veins continue into the arcuate → interlobar → segmental → main renal vein → inferior vena cava.
Drawing. Normally, with CDK, the renal vessels are traced to the capsule (1, 2, 3). The main renal artery enters through the renal hilum; accessory arteries from the aorta or iliac artery may approach at the poles (2).
Drawing. Ultrasound of a healthy kidney: along the base of the pyramids (corticomedullary junction) linear hyperechoic structures with a hypoechoic track in the center are identified. These are arcuate arteries, which are mistakenly regarded as nephrocalcinosis or stones.
Video. Arc-shaped arteries of the kidney on ultrasound
Juxtamedullary blood flow pathway
O.A. Kaplunova
Federal State Budgetary Educational Institution of Higher Education "Rostov State Medical University" of the Ministry of Health of the Russian Federation; Rostov-on-Don, Russia
The dissertation of the private associate professor of operative surgery and topographic anatomy of the Imperial Kazan University Vasily Zakharovich Golubev (1894) “On the blood vessels in the kidneys of mammals and humans” [1] was carried out in the histological laboratory under the direction of K.A. Arnstein. This fundamental work contained the most complete and reliable presentation of the patterns of distribution of the thinnest blood vessels in the cortex and medulla of the kidney and their relationships. Research by V.Z. Golubev contributed to the further development of the doctrine of microangioarchitecture of the kidneys and their potential resources
First of all, V.Z. Golubev deciphered the structure of two capillary networks in the renal cortex: glomerular and peritubular. Glomerular networks form the afferent glomerular arterioles, which branch from the interlobular arteries. Lateral branches without vascular glomeruli also branch off from the latter, forming the peritubular capillary network of the cortex. Internal perforating arteries, which have few or no glomeruli, begin from the arcuate or interlobular arteries and direct blood along the shortest path from the arcuate arteries to the vascular network of the fibrous or fatty capsule. These data are confirmed by modern studies using microcomputed tomography [2].
The renal medulla is supplied with blood by true and false straight arterioles. V.Z. Golubev showed that true straight arterioles arise directly from the arcuate arteries or from the proximal parts of the interlobular arteries, and false straight arterioles begin from the efferent arterioles of the juxtamedullary glomeruli. Having penetrated the medulla, straight arterioles form vascular bundles, which descend to the renal papilla and branch into capillaries that intertwine the urinary tubules. As part of the direct vessels of the medulla V.Z. Golubev identified true straight arterioles, false straight arterioles and straight venules of the pyramids.
The data of V.Z. are characterized by particular novelty. Golubev about “new wonderful networks” - glomerular capillary formations located along the arcuate arteries in the boundary layer of the kidney. Unlike the glomeruli of the renal corpuscles, they are not surrounded by capsules, and their afferent and efferent arterioles are located at opposite poles. V.Z. Golubev attributed these formations to the perivascular bed. In the border (juxtamedullary) zone of the kidney, “new wonderful networks” connect the vessels of the cortex and medulla, and regulate renal blood flow. V.Z. Golubev wrote: “Representing a reserve blood reservoir, a wonderful network can, by the play of muscles embedded in the walls of its branches and the adductor and efferent trunk, move the blood forward or hold it in place, depending on the circumstances, or in a roundabout lateral way direct it to the nearest area and thereby, to a certain extent, eliminating the harm of impaired blood circulation.”
Of great importance in the mechanism of hemocirculation in the kidney V.Z. Golubev assigned true direct arterioles, which “in addition to their physiological significance, can be regulators of blood circulation, being equipped with wonderful networks.”
Data from V.Z. Golubev about vascular anastomoses of the corticomedullary (juxtamedullary) zone of the kidney, direct arterioles of the medulla and his ingenious assumption about their physiological significance in the regulation of intrarenal circulation were confirmed by the experimental works of J. Trueta et. al. [3]. The authors found that the juxtamedullary glomeruli, vasa recta and associated vascular elements constitute a cerebral shunt through which blood, flowing from the cortex, goes from the renal arteries to the renal veins. The juxtamedullary shunt, according to the authors, includes: the proximal part of the interlobular arteries, the first branches of the interlobular arteries, the afferent and efferent vessels of the juxtamedullary glomeruli and the glomeruli themselves, straight arterioles in the medulla, loops of straight arterioles, intertubular capillaries forming part of the system of straight arterioles, straight venules, proximal part of interlobular veins.
Under normal conditions, only 20% of the renal blood passes through the juxtamedullary shunt. During anxiety, heavy physical activity, and especially in pathological conditions (cardiogenic or other shock), most of the blood (up to 80%) is discharged through the cerebral vessels. Significant intensification and duration of shunting lead to severe disruption of the blood supply to the superficial layers of the renal cortex and acute renal failure [4].
The regulation of renal blood circulation involves adaptive formations in large arteries that protrude into the lumen of the vessels [5, 6, 7].
The peculiarity of renal blood circulation is due to the ability of autoregulation, which results in intrarenal redistribution of blood flow: a decrease in the superficial layers and an increase in the deep layers of the kidney [8]. Autoregulation of renal blood flow is perfect, multicomponent and based on the functioning of the “macula densa - glomerular filtration rate” mechanism. The regulation of glomerular blood flow involves the renin-angiotensin-aldosterone and kallikrein-kinin-prostaglandin systems, the nervous system and atrial natriuretic factor. As a result of the operation of such a multifactorial system, even significant fluctuations in blood pressure do not have a noticeable effect on renal blood flow.
The kidneys take part in the regulation of systemic blood pressure and as an endocrine organ [9, 10]. Some renal hormones have a vasoconstrictor effect (vasopressor systems), others have a vasodilator effect (vasodepressor systems). A characteristic feature of these systems is that they act not only on individual target organs, but also directly on the kidney (local or tissue regulators). The juxtaglomerular apparatus, which secretes renin, and the interstitial cells of the medulla, which produce prostaglandins, closely interact in the regulation of renal blood flow, distributing it between the cortex and medulla [10].
The most universal reaction of the vessels of the renal cortex is vasoconstriction [4]. It is observed during orthostasis, emotional stress, physical activity, hypoxia, etc. The afferent vessels of the glomeruli are also susceptible to vasoconstriction. Blood flow in the renal medulla increases in this case [10]. Since the production of depressor substances (prostaglandins) is directly dependent on the level of medullary blood flow, this reaction can be considered as one of the important factors preventing excessive vasoconstriction of the vessel and stabilizing high blood pressure [8]. The vasospasm of the renal cortex observed with most exposures has an adaptive significance, which consists in eliminating actually existing or preventing expected hypovolemia and hypotension. In this case, in the kidneys, the bulk of the blood is discharged through the J. Trueta shunt. However, this reaction is not always advisable, since when it is repeated many times, cortical necrosis develops. The kidneys, as if sacrificing their own blood circulation, maintain conditions for the blood supply to other organs, normalizing general blood circulation [4].
In cardiovascular and renal diseases, nephrosclerosis usually occurs [11, 12]. At a certain stage of the morphogenesis of nephrosclerosis of any etiology, a block of renal blood flow is formed, which can be localized at the level of different kidney structures: glomeruli - in chronic glomerulonephritis and amyloidosis, afferent arterioles - in arterial hypertension, peritubular capillaries - in chronic nephritis. A block of blood flow, regardless of its location, entails the development of hypoxic diffuse sclerosis of the stroma and atrophy of nephron structures.
The lack of effective regulation of cerebral blood flow in the kidneys during arterial hypertension contributes to extravasation and the development of hyalinosis of the medulla stroma [13]. With sclerosis of the renal medulla, a reduction and block of the juxtamedullary shunt is observed [14], which, in turn, potentiates the damaging effect of arterial hypertension on the structures of the cortical substance due to increased hemodynamic load [12].
Our studies [7] have shown that the architecture of the vessels of the microvasculature of the kidneys in cardiovascular diseases is characterized by a decrease in the diameters of the vascular glomeruli, a decrease in the glomerular mass in the cortex and the total number of glomeruli in the kidney. Desolation and deformation of the peritubular capillaries, expansion and deformation of the straight arterioles of the medulla are also observed. These changes are most pronounced in renovascular hypertension, a combination of hypertension with chronic coronary heart disease, and in hypertension compared with coronary heart disease and atherosclerosis of the abdominal aorta.
It should be noted that in the studied cardiovascular diseases, adaptive processes in the vessels of the intraorgan arterial bed of the kidneys are associated with a decrease in the relative content of arterial vessels in the cortex and an increase in them in the medulla of the kidneys, a decrease in the diameters of the vascular glomeruli of the subcapsular and intracortical renal corpuscles and an increase in the juxtamedullary glomeruli. The diameter of the direct medullary arterioles also increases. These changes in the intraorgan arterial vessels of the kidneys are especially pronounced in acute coronary heart disease and hypertension, and indicate increased juxtamedullary shunting (Fig. 1-b, 2-b, 3). In chronic cardiovascular diseases (chronic ischemic heart disease, hypertension in combination with chronic ischemic heart disease), when there is long-term exposure to changed conditions of hemocirculation, changes were found in the microcirculatory bed of the kidneys according to the type of excessive plethora - a manifestation of long-term adaptation (Fig. . 4). With sclerotic and destructive changes in the intraorgan arterial bed of the kidneys, the relative content of arterial vessels in all zones of the kidneys significantly decreases, which is obviously associated with the absence of juxtamedullary shunting and excessive plethora and indicates a failure of adaptation processes (Fig. 1-c, 5.) [15].
Figure 1. Arterial vessels in the middle zone of the cortex: A – in the kidney of a 37-year-old man, control group; B – collapsed peritubular capillaries in the kidney of a 68-year-old man, chronic ischemic heart disease; C – sclerotic and deformed glomeruli and vessels in the kidney of a 71-year-old woman, vasorenal hypertension (atherosclerosis of the renal artery). Staining with erythrosine according to G.V. Kovalevsky. Volume 15, approx. 7.
Figure 2. Arterial vessels of the medulla: A – in the kidney of a 23-year-old man, control group; B — dilatation and overflow of blood into the direct arterioles in the kidney of a 49-year-old man, acute coronary heart disease. Erythrosine staining according to G.V. Kovalevsky. Volume 15, approx. 7.
Figure 3. Dilated straight arterioles, the lumens of which are filled with black ink, and venules with blood cells (shown by arrows) in the renal medulla of a 58-year-old man. Chronic ischemic heart disease. Injection of blood vessels with black ink. Weigert staining. About. 40, approx. 10.
Figure 4. Straight arterioles, the lumens of which are filled with black ink, and dilated venules with blood cells (shown by arrows) in the renal medulla of a 41-year-old man. Stage 2 hypertension. Injection of blood vessels with black ink. Weigert staining. About. 40, approx. 10.
Figure 5. A section of the renal medulla of a 71-year-old woman with chronic ischemic heart disease, secondary hypertension, and atherosclerosis of the abdominal aorta. Foci of sclerosis of the medulla (1) and deformation of the straight arterioles (2). Staining according to van Gieson. About. 16, approx. 10.
So, in acute cardiovascular diseases, the importance of the juxtamedullary blood flow pathway increases, and juxtamedullary shunting is observed. In chronic cardiovascular diseases, juxtamedullary shunting initially increases, and in the later stages of the disease, sclerosis of the renal medulla causes a reduction and block of the juxtamedullary blood flow path, both the juxtamedullary and cortical blood flow paths deteriorate, which leads to disruption of the adaptation of the intraorgan arterial bed of the kidneys.
Based on the data obtained, we established a pattern associated with the process of adaptation of the intraorgan arterial bed of the kidneys both to age-related changes in hemocirculation and to the studied cardiovascular diseases. It should be noted that the same morphological structures are involved in this process - the tunica media and elastic membranes of intraorgan arteries, muscle-elastic cushions in the places of branch arteries and afferent arterioles of the glomeruli of intracortical and juxtamedullary renal corpuscles, as well as the changing diameters of afferent and efferent vascular arterioles glomeruli of various zones of the renal cortex. For each age group and for each disease studied, the degree of change in these structures affects the zonal redistribution of blood flow in the kidneys [16].
Modern information about the pathways of renal hemocirculation, prepared by the research of V.Z. Golubev, allow us to explain the mechanism of development of various pathological conditions: acute blood loss [17], hydronephrosis [18], vasorenal hypertension, glomerulonephritis, pyelonephritis [8], nephrourological diseases [19], cardiogenic shock [20, 21], urolithiasis [22] , cardiorenal syndrome in acute coronary heart disease [23], arterial hypertension [7, 24, 25], sudden coronary death [26], etc. Thus, V.Z. Golubev created the scientific basis for the study of renal microangioarchitecture. The principles set forth in his dissertation anticipated the appearance of works studying juxtamedullary blood flow and renal hemomicrocirculation in health and disease.
Literature
- Golubev V.Z. About blood vessels in the kidney of mammals and humans. Diss. Kazan; 1894: 110.
- Marsh DJ, Postnov DD, Rowland DJ, Wexler AS, Sosnovtseva OV, Holstein-Rathlou NH. Architecture of the rat nephronarterial network: Analysis with micro-computed tomography. Am J Physiol Renal Physiol. 2017;313(2):F351-F360. DOI: 10.1152/ajprenal.00092.2017
- Trueta J, Barclay AE, Daniel PN, et al. Studies of the renal circulaƟ on. Oxford: Charles Thomas, Springfield; 1947.
- Suchkov V.V. Cortical and cerebral circulation of the kidneys during pressor and depressor sinocarotid reflexes. Cardiology. 1984;24(6):24-28.
- Alekseevskikh Yu.G. To some histological features of the structure of arteries and veins of the kidneys in humans. Arch. pathol. 1969;31(6):42-45.
- Makarenko N.Yu. Age-related features of the intraorgan vessels of the kidney, factors that determine them. Basic sciences – medicine and healthcare. 1989;1:87-88.
- Kaplunova O.A. Blood vessels of the kidneys. Rostov-on-Don: Nauka-Spectrum; 2008.
- Serov V.V. Kidneys and arterial hypertension. M.: Medicine; 1993.
- Shkhvatsabaya I.K., Nekrasova A.A., Ustinova S.E. and others. Pressor and depressor humoral systems in hypertension, occurring with a labile and stable increase in blood pressure. Arterial hypertension. M.: Medicine; 1980.
- Serov V.V., Varshavsky L.A., Kupriyanova L.A. Immunopathology of the kidneys. M.: Medicine; 1983.
- Serov V.V., Yargin S.V. Morphological and pathogenesis of nephrosclerosis: clinical and morphological analysis. Therapeutic archive. 1986;58(8):4-9.
- Yargin S.V. Nosological features of the morphogenesis of nephrosclerosis. Pathology archive. 1986;7:55-63.
- Postnov Yu.V. Kidney in chronic arterial hypertension, and “switching” and the role of the renal medulla in its development. Cardiology. 1979;21(12):30-38.
- Krotovsky G.S., Paltsev M.A., Pokrovsky A.V. and others. Mathematical modeling of the severity of nephroangiosclerosis in vasorenal hypertension. Surgery. 1989; 6:23-27.
- Kaplunova O.A. Morphofunctional characteristics of intraorgan arterial vessels of the kidneys in normal conditions and in some cardiovascular diseases. Journal of Basic Medicine and Biology. 2014;2:25-29.
- Kaplunova O.A. Morphological aspects of age-related adaptation of the arterial vessels of the human kidneys in some cardiovascular diseases. Medical Bulletin of the South of Russia. 2014; 2: 65-70.
- Vorobiev A.I., Gorodetsky V.M., Shulutko E.M. etc. Acute massive blood loss. M.: GEOTAR-Media; 2001.
- Asfandiyarov F.R., Abdulkhakimov E.R. Microvasculature of the kidneys in hydronephrosis according to laser Doppler intraoperative flowmetry and morphological study. Bulletin of the Volgograd State. honey. university. 2009;3(31):32-34.
- Olkhova E.B. Intrarenal arteriovenous shunting in children. Ultrasound and functional diagnostics. 2004;2:67-77.
- Barbarash L.S., Popkov A.N., Kheraskov V.Yu., Plotnikov G.P., Shukevich D.L., Grigoriev E.V. The effectiveness of renal replacement therapy for cardiogenic shock complicated by multiple organ failure. General resuscitation. 2011;7(5):32. DOI: 10.15360/1813-9779-2011-5-32
- Van den Akker JPC, Bakker J, Groeneveld ABJ, den Uil CA4. Risk indicators for acute kidney injury in cardiogenic shock. J Crit Care. 2019;50:11-16. DOI: 10.1016/j.jcrc.2018.11.004
- Asfandiyarov F.R. Structural transformations of the renal artery system at the stages of prenatal ontogenesis, aging and pathological conditions. Author's abstract...doctoral dissertation. Saratov; 2011: 42.
- Mukhin N.A., Moiseev V.S., Kobalava Zh.D. Cardiorenal interactions: clinical significance in the pathogenesis of diseases of the cardiovascular system and kidneys. Ter. Archive. 2004;6:5-10.
- Gogin E.E. Hypertension: basics of pathogenesis, diagnosis and choice of treatment. Consilium medicum. 2004;6(5):324-330.
- Ruiz-Hurtado G, Ruilope LM. Microvascular injury and the kidney in hypertension. Hipertens Riesgo Vasc. 2018;35(1):24-29. DOI: 10.1016/j.hipert.2017.03.002
- Sokolova R.I., Volkov V.I., Wichert A.M. and others. Features of microcirculation in the kidneys during sudden cardiac death. Pathology archive. 1986; 48 (8): 44-49.
The article was published in the journal “Bulletin of Urology” No. 1 2021, pp. 45-52
Magazine
Urology Bulletin No. 1 2019
Comments
To post comments you must log in or register
Doppler of renal vessels is normal
The normal diameter of the renal artery in adults is 5 to 10 mm. If the diameter is <4.65 mm, an accessory renal artery is likely present. When the diameter of the main renal artery is <4.15 mm, an additional renal artery is almost always present.
The renal artery should be assessed at seven points: at its exit from the aorta, in the proximal, middle and distal segments, as well as the apical, middle and lower segmental arteries. We evaluate peak systolic (PSV) and end-diastolic (EDV) blood flow velocities, resistivity index (RI), acceleration time (AT), acceleration index (PSV/AT). See Vascular Doppler for more details.
The normal spectrum of the renal arteries has a pronounced systolic peak with antegrade diastolic flow throughout the cardiac cycle. In adults, the normal PSV on the main renal artery is 100±20 cm/sec, EDV is 25-50 cm/sec, in young children PSV is 40-90 cm/sec. In segmental arteries, PSV drops to 30 cm/sec, in interlobar arteries to 25 cm/sec, in arcuate arteries to 15 cm/sec and interlobular arteries to 10 cm/sec. RI at the renal hilum <0.8, RI at the intrarenal arteries 0.34-0.74. In a newborn, RI on the intrarenal arteries reaches 0.8-0.85, by 1 month it drops to 0.75-0.79, by 1 year to 0.7, in adolescents 0.58-0.6. Normal PI is 1.2-1.5; S/D 1.8-3.
Drawing. The normal spectrum of the renal arteries is high systolic peak, antegrade diastolic flow, low peripheral resistance - RI is normal <0.8.
Drawing. The spectrum of renal vessels in newborns: renal artery - pronounced systolic peak and antegrade diastolic flow (1); high resistance in the intrarenal arteries is considered normal for newborns - RI 0.88 (2); renal vein - antegrade flow with a constant speed throughout the entire cardiac cycle, minimal respiratory fluctuations (3).
Doppler for renal artery stenosis
Renal artery stenosis can be found in atherosclerosis or fibromuscular dysplasia. With atherosclerosis, the proximal segment of the renal artery is most often affected, and with fibromuscular dysplasia, the middle and distal segments are most often affected.
Direct signs of renal artery stenosis
The aliasing indicates the location of the turbulent high-speed flow where measurements should be made. In the area of stenosis PSV >180 cm/sec. In young people, the aorta and its branches may normally have a high PSV (>180 cm/sec), while in patients with heart failure, the PSV is low even in the area of stenosis. These features are leveled by the renal-aortic ratio RAR (PSV in the area of stenosis/PSV in the abdominal aorta). RAR for renal artery stenosis >3.5.
Table. Criteria for renal artery stenosis on ultrasound
| Renal artery stenosis | PSV at the site of stenosis | RAR |
| Norm | <180 cm/sec | <3,5 |
| <60% | >180 cm/sec | <3,5 |
| ≥60% | >180 cm/sec | ≥3,5 |
| Occlusion | No signal | — |
Indirect signs of renal artery stenosis
Direct criteria are preferable; diagnosis should not be based solely on indirect signs. In the post-stenotic region, the flow attenuates - tardus-parvus effect. With renal artery stenosis on the intrarenal arteries, PSV is too late (tardus) and too small (parvus) - AT >70 ms, PSV/AT <300 cm/sec². The significant difference between the two kidneys is alarming: RI >0.05 and PI >0.12.
Table. Criteria for renal artery stenosis on ultrasound
| Signs | Main renal artery | Segmental or interlobar arteries |
| Direct | PSV >180 cm/sec, RAR >3.5 | |
| Indirect | AT >70 ms, AI <300 cm/sec² | |
| Combination | PSV in interlobar arteries <15 cm/sec, RIR (PSV renal artery at the site of stenosis/PSV interlobar artery) >5 | |
Drawing. A 60-year-old female patient with refractory arterial hypertension. PSV on the abdominal aorta 59 cm/sec. In the proximal part of the RAA with CDK aliasing (1), PSV is significantly increased 366 cm/sec (2), RAR 6.2. In the middle segment of the PPA with CDK aliasing, PSV 193 cm/sec (3), RAR 3.2. On segmental arteries without a significant increase in acceleration time: upper - 47 ms, middle - 93 ms, lower - 33 ms. Conclusion: Stenosis in the proximal part of the right renal artery.
Drawing . Patient with acute renal failure and refractory arterial hypertension. Ultrasound of the abdominal aorta and renal arteries is difficult due to gas in the intestine. On the segmental arteries on the left RI is about.68 (1), on the right RI is 0.52 (2), the difference is 0.16. The spectrum of the right segmental artery has a tardus-parvus shape - acceleration time is increased, PSV is low, the apex is rounded. Conclusion: Indirect signs of stenosis of the right renal artery. CT angiography confirmed the diagnosis: at the mouth of the right renal artery there are atherosclerotic plaques with calcification, moderate stenosis.
Drawing. Patient with arterial hypertension. PSV in the aorta is 88.6 cm/sec (1). In the proximal part of the RPA there is aliasing, PSV 452 cm/sec, RAR 5.1 (2). In the middle section of the PPA there is eleasing, PSV 385 cm/sec, RAR 4.3 (3). In the distal part of the RCA, PSV is 83 cm/sec (4). On the intrarenal vessels tardus-parvus the effect is not determined, on the right RI is 0.62 (5), on the left RI is 0.71 (6), the difference is 0.09. Conclusion: Stenosis in the proximal part of the right renal artery.
Ultrasound examination of the renal arteries
Ultrasound diagnostics is a medical imaging method that began to be used more than 40 years ago. Currently, medicine can no longer imagine its existence without this method. Ultrasound examinations are now ubiquitous.
Some diseases go unnoticed in the initial stages, and a late visit to a doctor can complicate the entire treatment process, which, moreover, will not always be effective. Therefore, the ultrasound diagnostic method quickly gained popularity. Ultrasound is used in a wide variety of fields of medicine, which is facilitated by its fairly high information content and the possibility of repeated examinations. For diagnostic purposes, it is used to identify diseases of the abdominal and kidney organs, pelvic organs, thyroid gland, mammary glands, lymphatic system, heart, blood vessels, in obstetric and pediatric practice. Ultrasound diagnostics has almost 100% sensitivity in detecting focal organ damage, and 93% in detecting cancer. Studies of the condition of the renal arteries (RA) are also considered extremely important, since disturbances in renal blood flow can cause various pathologies, especially in persons suffering from arterial hypertension. Ultrasound of the renal arteries is performed to exclude renovascular hypertension. When the renal artery narrows, poorly controlled hypertension occurs. Increased blood pressure at a young age or with systemic atherosclerosis is also an indication for ultrasound of the renal arteries. The duplex scanning method, combined with color Doppler mapping of the color flow, allows a non-invasive way to assess blood flow in the renal arteries (RA). Currently, ultrasound criteria have been developed for the diagnosis of hemodynamically significant stenoses (narrowings) of the VA; the feasibility of using DS during reconstructive operations on the VA, during kidney transplantation, as well as in the postoperative period to assess the effectiveness of the interventions performed has been shown. The sensitivity and specificity of the duplex scanning method in detecting hemodynamically significant VA lesions are 99-100% and 98%, respectively. The emergence of a new class of ultrasonic devices with high resolution and allowing the production of DS with color flow in high-speed and energy modes has opened up new prospects in the study of the vascular bed of the kidney and made it possible to study and evaluate renal blood flow not only in the main trunk of the renal vein, but also in segmental ones. , interlobar arteries, as well as in the arteries of the renal cortex. Methodology for ultrasound examination of the renal arteries For better visualization of the renal arteries, it is recommended to conduct an ultrasound examination in the morning, on an empty stomach, to prevent the accumulation of intestinal gases during the day and after meals. The last meal should be the evening before the test. Longer fasting is not recommended due to the possible development of intestinal flatulence. When preparing for the study, it is advisable to avoid smoking and chewing gum. Oral administration of a small amount of medications necessary for the patient is allowed. However, in the vast majority of patients, examination of the VA (especially its distal parts) is possible without special preparation. Ultrasound Doppler of the Renal Veins with Color Doppler The venous system of the kidney largely coincides with the arterial one, only, unlike arteries, the renal veins are connected to each other and to neighboring venous plexuses. To assess the renal veins, ultrasound dopplerography with color duplex scanning is used. Using color mapping and continuous Doppler ultrasound, ultrasound detects pathologies of the renal arteries with high reliability. Doppler ultrasound is absolutely safe for the kidneys and blood vessels. After the ultrasound diagnosis, the doctor will issue a conclusion and recommend further actions for you.
Ultrasound diagnostics doctor of the 1st category, candidate of medical sciences, cardiologist – Slutskaya Natalya Vladimirovna
Doppler of renal veins
The left renal vein passes between the aorta and the superior mesenteric artery. The aortomesenteric “tweezers” can compress the vein, leading to venous renal hypertension. In a standing position, the “tweezers” compress, and in a lying position, they open. With Nutcracker syndrome, outflow through the left testicular vein is difficult. This is a risk factor for the development of left-sided varicocele.
Due to compression, the spectrum of the left vein is similar to the portal vein - spectrum above the baseline, constant low speed, contour in smooth waves. If the ratio of the diameter of the left vein in front of and in the narrowing zone is more than 5 or the flow rate is less than 10 cm/sec, we conclude that the venous pressure in the left kidney is increased.
Task. On ultrasound, the left renal vein is dilated (13 mm), the area between the aorta and the superior mesenteric artery is narrowed (1 mm). Blood flow in the area of stenosis at high speed (320 cm/sec), reverse blood flow in the proximal segment. Conclusion: Compression of the left renal vein by aortomesenteric “tweezers” (Nutcracker syndrome).
Compression of the renal vein is possible due to its abnormal location behind the aorta. The diameter ratio and flow rate are evaluated according to the above rules.
The nature of the blood flow in the right renal vein approaches that of the caval vein. The shape of the curve changes when you hold your breath and can be flatter. Blood flow speed is 15-30 cm/sec.
Take care of yourself, Your Diagnosticer !
Tags: Doppler lectures kidneys ultrasound