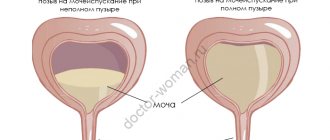
Symptoms of urethral stricture
Urethral stricture is manifested by a gradual weakening of the pressure of the urine stream, difficult, sometimes intermittent urination. In later stages, there is a feeling of incomplete emptying of the bladder and the presence of residual urine.
As the disease progresses and the lumen of the urethra narrows, the phenomena of chronic urinary retention increase, up to the impossibility of independent urination.
Then events develop rapidly - severe bursting pain occurs in the lower abdomen, and the groaning sufferer is taken by ambulance to the nearest hospital, where a urologist inserts a tube into his bladder (cystostomy) through a puncture in the abdomen.
Fortunately, the disease does not always reach this tragic ending - in many cases, the patient encounters a qualified urologist in his life who knows the methods of diagnosing and surgical treatment of urethral stricture.
Minimally invasive methods for treating anterior urethral strictures
V.L. Medvedev1,2, Yu.N. Medoev2, V.V. Mitusov3 1 Federal State Budgetary Educational Institution of Higher Education “Kuban State Medical University” of the Ministry of Health of the Russian Federation; Krasnodar, Russia 2 State Budgetary Healthcare Institution “Research Institute – Regional Clinical Hospital No. 1 named after. prof. S.V. Ochapovsky" Ministry of Health of the Krasnodar Territory; Krasnodar, Russia 3 Federal State Budgetary Educational Institution of Higher Education "Rostov State Medical University" of the Ministry of Health of the Russian Federation; Rostov-on-Don, Russia Contact author: Medoev Yuri Nikolaevich Tel.: +4; e-mail
Introduction
Urethral stricture (SU) in men has been known to urologists for a long time and remains one of the most common diseases in urological practice. The presented literature review is a reflection of both modern views and historical facts on such methods of its treatment as bougienage, internal urethrotomy and stenting. The literature search was performed using Medline, PubMed, and Embase databases. Data were assessed using the evidence scale established by the International Consultation on Urological Diseases (ICUD) at the Oxford Centre.
The simplest and most well-known method of treating SU is its bougienage (palliative method) using various instruments. A qualitatively new approach in the treatment of stricture disease should be considered in 1974, when Sachse H. used internal optical ureterotomy (IOUT) with dissection of the stricture zone [1]. Despite the fact that open reconstruction is considered a radical method of treating strictures, VOUT has been recognized as the standard of care in the treatment of short anterior urethral strictures, because urethroplasty requires the experience of the surgeon, the availability of an operating room and involves a longer postoperative period and rehabilitation of the patient [2,3].
A number of articles since 1990 have described many successful interventions using VOOT. However, several modifications were made to the technique of dividing the stricture, such as using a laser instead of a urethrotome and placing a stent in the anterior urethra. But this did not lead to significant improvements in the results of treatment of SU. To a greater extent, the effectiveness of treatment was associated with assessment of the criteria for SU, its location and extent. It is also necessary to take into account symptoms, uroflowmetry indicators, urethrography data, and the presence of urinary tract infection.
VUT/dilatation of anterior urethral stricture
Studies have shown that in America, 92.8% of urologists, according to some sources, and 85.6% - according to others, prefer to use bougienage and/or dissection of strictures in the anterior urethra [4,5]. This preference for VOUT/bougienage is associated with the ease of performing the manipulation, minimal costs, and the ability to perform it under local anesthesia on an outpatient basis, which does not require long-term rehabilitation of the patient [6,8].
The purpose of this procedure is to perform a minimally invasive intervention designed to improve urethral patency with minimal complications [9]. For carefully selected patients with optimal stricture characteristics (predominantly bulbar stricture, <1 cm, mild), the stricture cure rate (SCR) is 50-70%. For such patients, urethrotomy is the treatment of choice for the disease [10-12]. It should be emphasized that such PIS is significantly lower than when performing anastomotic urethroplasty (90-95%) [3,13-16], but urethrotomy is distinguished by its ease of performance and less trauma for the patient. The incidence of complications after VOUT ranges from 6 to 22% and includes pain, bleeding, exacerbation of urinary tract infection, erectile dysfunction (ED) [10,12,17]. A number of studies have proven that repeated VOUT/bougienage in patients who have previously undergone this procedure significantly increases the risk of developing a relapse of the disease with an increase in the extent of damage to the urethra [10,12,17-20]. Based on the use of a variety of cutting techniques, which range from blind dilatation to VOUT, where the instrument is a cold knife, hot knife or laser, it was concluded that VOUT is too often used in cases where urethroplasty has would have a greater effect. To a lesser extent, the effectiveness of VOUT is influenced by the age of the patients, the length and localization of the SU, and the primary or recurrent condition. [19].
As a result of a 1997 study by Steenkamp JW, Heyns CF, de Kock ML et al. [11] the study proved the effectiveness of VOUT and bougienage of SU, which previously had only level 3 evidence (level 1). In two carefully studied groups of 104 and 106 patients, bougienage and VOT were performed. This study showed that for strictures <2 cm, it is recommended to perform internal bougienage, for strictures >4 cm, urethroplasty, and trial VOUT for stricture lengths of 2-4 cm. Patients were studied for the time of disease recurrence, and the establishment of AIS over 48 months. Early recurrence (<3 months) with repeated bougienage/dissection was found to be a poor predictor of 30%. A similar percentage of relapse occurred after 24 months of observation, 0% by 48 months. If stricture recurrence occurred after more than 6 months, the need for repeat WHUT was 40%. Recurrent urethral strictures that underwent a 3rd procedure of dissection or bougienage resulted in a 100% recurrence rate [17].
Pansadoro V., Emiliozzi P. analyzed 224 patients who underwent VOT for short strictures with a follow-up period of 98 months. The AIS value was 32%, but it varied greatly and depended on the length, location, diameter of the stricture, its primary or recurrent formation. Strictures with a length of 1 cm had PIS rates of 71% and 18%, respectively, and in terms of diameter and narrowing relative to the normal lumen, >15% and <15% had PIS rates of 69% and 34, respectively [10].
The second large study was carried out by Albers P., Fichtner J., Brühl P. et al. on a group of 580 patients and took 3 years. AIS rates were more than 55% effective when patients had a stricture in the bulbar urethra. Strictures greater than 1 cm in length treated with VOT, multiple strictures, and penile strictures had higher recurrence rates. Based on this, the authors concluded that urethroplasty should be performed in case of a recurrent condition after repeated bougienage or incision of the stricture [12].
It has been shown that the best results are achieved with a stricture length of 15F. VOUT/bougienage or urethroplasty can be performed as first-line therapy when the stricture is located in the penile or penile-bulbar region due to the low PIS rates when performed, and when performed repeatedly (>2) they should be considered disease.
Santucci R., Eisenberg L. concluded that internal urethrotomy has a lower success rate than previously stated. They conducted a retrospective medical study on 134 patients who underwent internal urethrotomy from 1994 to 2009. Thirty-six patients with complex strictures and 24 who themselves refused this treatment were excluded. AIS rates after the 1st, 2nd, 3rd, 4th, 5th internal urethrotomy were 8%, 6%, 9%, 0% and 0%, and the average period before relapse was 7, 9 , 3, 20 and 8 months [18]. Different effectiveness assessments and different research standards on the admissibility of these techniques do not always allow one to compare one result with another.
Repeated WOUT/bougienage
In patients with the general characteristics of stricture recurrence (single, 6 months), the success rate of repeat VOTS is 9%-53% (grade 2-3) [10,17,18,21,22]. For longer, multifocal lesions and distal penile strictures, dissection/bougienage is usually not performed [2,23]. VOT is not indicated if a relapse of the disease occurs less than 3 months after the 3rd VOT. Urethroplasty is indicated for such patients [2,3,12,17,21].
Side effects of VOUT
The most common types of complications after VOT are hemorrhage and the formation of perineal hematoma (in 20% of cases) [17]. According to other sources, edema (13%), formation of false ducts (10%), rectal perforation, epididymo-orchitis, meatus stenosis and urinary incontinence (9%), hyperthermia (3.6%), hematoma (3.4%) also occur. , bacteremia (2.7%), urosepsis (2.1%) and abscess formation (1.4%). Data on ED usually range from 2-10% [24], however Schneider T, et al. [25] conducted a study on 68 patients without ED before surgery and found that after VOUT, only 1 patient complained of ED in the postoperative period.
Profitability
Some studies addressing the issue of cost-effectiveness have shown that performing simple urethrotomy for anterior urethral strictures is cost-effective in cases where success can be predicted in >35%-50% of cases. Otherwise, primary urethroplasty is more beneficial than repeat urethrotomy. Wright et al. found that the best option for short strictures in the bulbar region is to perform urethroplasty rather than endoscopic intervention. In the presence of a long stricture, where the predicted success is <35%, urethroplasty should be the primary method of choice and treatment of the disease, which has been proven endoscopically [26]. Two identical studies have shown that urethroplasty is most cost-effective when VUT or bougienage results in stricture recurrence after primary surgery [27–29].
These studies only take into account the financial issue of these procedures in developed countries and are not always applicable to developing countries. Articles have been published from Nigeria, which present the results of treating a large number of strictures with insufficient financial security. In 134 men treated from 1993 to 1996. using the method of urethrotomy and subsequent self-bouging, the rate of stricture recurrence was 17%, and after urethroplasty - 22%. It turns out that in this case, urethrotomy is 10 times cheaper than urethroplasty, and the surgeon himself is more protected from infection with the human immunodeficiency virus (HIV) (level 3) [30].
Laser urethrotomy
One of the options for performing VOUT is to dissect the urethral stricture area with a laser. This allows not only to cut the narrowing zone, but also to cause the evaporation of tissue structures, which has been widely used over the past 30 years. Argon, excimer, diode and other lasers were mainly used. Over the past 10 years, the surgical arsenal has been replenished with holmium and thulium lasers. There are no level 2 data assessing them, only a few level 3 articles. Thus, there is no data yet on the advantage of laser urethrotomy over other types of dissection of stricture zones.
HEU in combination with self-dilatation
Previously, patients were offered self-catheterization after undergoing VOWT to prevent relapse of the disease. Many patients found it painful, unpleasant, burdensome, with the risk of infection and abscess development, other injuries leading to stricture recurrence and the formation of false passages [30,31].
Culty T., Boccon-Gibod L. [23] found that performing urethral dilation has a negative impact on patients who are planning to undergo anastomotic urethroplasty. The success rate in cases where premature dilatation was abandoned was 90% and 60% in patients who underwent it.
HEU combined with chemicals
Some articles devoted to VOUT note the advisability of using targeted medication for VOUT. [23,24]. According to level 2 articles, 40 patients with short bulbar stricture (average, about 0.75 mm) underwent VOWT alone and VOWT in combination with mitomycin B (MBC) injections [33]. The results of relapse with VOWT alone were 50%, and with VOWT combined with mitomycin injections - 10%. This was a carefully selected group of young patients with a very limited time frame for the study. The same authors conducted studies in 50 patients who underwent VOWT alone and VOWT with urethral, subcutaneous triamcinolone. The authors found a decrease in relapse rates from 50% with VOUT to 21% with VOUT with urethral subcutaneous administration of triamcinolone. As in the first observation, this was a carefully selected group of young patients with a short bulbar stricture (<1.0 cm) and limited follow-up time [34].
Stenting of the anterior urethra
The concept of the possibility of stenting the anterior urethra for SU dates back to 1969 and found clinical application in 1989, when Milroy E., Allen A. [35] announced the “discovery” of a new method for treating urethral stricture. Designed for endovascular use, a self-expanding mesh stent made of stainless steel alloy was implanted in 8 patients with urethral stricture. During 8 months of research, all patients had good urethral caliber indicators. In later studies in 10 patients with the same stents, stricture recurrence was found in 30% at 24 months of observation, while the occurrence of post-micturition dribbling was noted in 50%.
De Vocht TF, van Venrooij GE, Boon TA assessed satisfaction with treatment results in patients over a period of 10 years after installation of the UroLume stent and found that only two out of 15 men were truly satisfied with the results. 4 patients had their stents removed (2 due to unbearable pain, 2 due to stent obstruction), 50% of patients developed urinary incontinence, and others reported discomfort with ejaculation [36].
The most common use of the UroLume stent was in men with recurrent short bulbar urethral strictures around the age of 50 years, despite the fact that they were optimal candidates for urethroplasty. Long-term observation showed that in 55% of cases such patients develop complications associated with stenting. 45% require surgery due to pain, post-vomiting dribbling, urinary incontinence, stent migration, stent obstruction, or recurrent stricture proximal or distal to the stent. Potency in such patients tends to decrease from time to time in 100% of observations (short studies), and in 45% (long-term observations up to 77 months) [37,38]. The stent itself can lead to loss of rigidity and elasticity of the urethra, which also requires surgical correction and reconstruction of the urethra [36,39,40].
The Memokath stent (a removable heat-resistant nylon stent), used in the treatment of prostatic urethral obstruction and detrusorosphincter dyssynergy in the posterior urethra, has been tested for stenting the anterior urethra. In a study of 92 patients who underwent bougienage/dissection of recurrent strictures in the bulbar region, who were subsequently fitted with a Foley catheter (n=29), and 63 patients with a Memokath stent, it was found that urethral patency was maintained 3.5 times longer than in the no-stent group, although all stents were subsequently removed. Side effects of stenting included urinary tract infection, hematuria, and penile pain. Stent migration was observed in 22% of patients. The ease of insertion and removal of the Memokath stent may be beneficial for bulbar urethral strictures in patients who cannot undergo urethral reconstruction for one reason or another. The use of such a technique for maintaining the passage of urine through the urethra during stricture disease requires further research [41].
Conclusion
The minimally invasive techniques used are widely used in surgical practice for MS, which is associated with ease of implementation and minimal costs of materials. The procedures can be performed under local anesthesia and do not require long-term rehabilitation of patients in the postoperative period. Good postoperative results are usually achieved when the patient selection criteria for each specific technique are met. A method such as placement of a urethral stent can be very useful for urethral strictures in patients who cannot undergo reconstructive surgery. VOWT with the introduction of mitomycin and VOWT with urethral percutaneous administration of triamcinolone increase the effectiveness of the technique by up to 50% compared to standard VOWT. The introduction into practice of modern lasers (argon, excimer, diode, holmium and thulium) has been used in the dissection and evaporation of strictures of the anterior urethra. All of the methods described above are inferior to standard urethroplasty, which is a radical method of treating structural disease, but can be used in patients who do not want to undergo open surgery, and in situations where it cannot be performed due to comorbidity of the pathology or for other reasons.
The study had no sponsorship. The authors declare no conflict of interest.
Literature
- Sachse H. Treatment of urethral stricture: Transurethral slit in view using sharp section. Fortschr Med. 1974;92(1):12-15.
- Kogan M.I., Krasulin V.V., Mitusov V.V., Shangichev A.V., Glukhov V.P. and others. Surgical treatment of strictures and obliterations of the urethra. Urology. 2015; 2:17-23.
- Glukhov V.P., Krasulin V.V. Urethral resection with end anastomosis in the surgical treatment of complicated urethral strictures in men. Kuban Scientific Medical Bulletin. 2009;4:78-82.
- Bullock TL, Brandes SB. Adult anterior urethral strictures: A national practice patterns survey of board certified urologists in the United States. J Urol. 2007;177(2):685-690.
- Anger JT, Buckley JC, Santucci RA, Elliott SP, Saigal CS; Urologic Diseases in America Project.. Trends in stricture management among male Medicare benefits: Underuse of urethroplasty? Urology. 2011; 77(2): 481-5. doi: 10.1016/j.urology.2010.05.055
- Steenkamp JW, Heyns CF, de Kock ML. Outpatient treatment for male urethral strictures–Dilation versus internal urethrotomy. S Afr J Surg. 1997;35(3):125-130.
- Altinova S, Turkan S. Optical urethrotomy using topical anesthesia. Int Urol Nephrol. 2007;39(2):511-512. doi: 10.1007/s11255-006-9046-0
- Ather MH, Zehri AA, Soomro K, Nazir I. The safety and efficiency of optical urethrotomy using a spongiosum block with sedation: A comparative nonrandomized study. J Urol. 2009;181(5):2134-8. doi: 10.1016/j. juro.2009.01.017
- Tonkin JB, Jordan GH. Management of distal anterior urethral strictures. Nat Rev Urol. 2009;6(10):533-538. doi: 10.1038/nrurol.2009.181
- Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: Long-term followup. J Urol. 1996;156(1):73-75.
- Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: A prospective, randomized comparison. J Urol. 1997;157(1):98-101.
- Albers P, Fichtner J, Brühl P, Müller SC. Long-term results of internal urethrotomy. J Urol. 1996;156(5):1611-1614.
- Barbagli G, De Angelis M, Romano G, Lazzeri M. Longterm followup of bulbar end-to-end anastomosis: A retrospective analysis of 153 patients in a single center experience. J Urol. 2007;178(6):2470-2473. doi: 10.1016/j. juro.2007.08.018
- Santucci RA, Mario LA, McAninch JW. Anastomotic urethroplasty for bulbar urethral stricture: Analysis of 168 patients. J Urol. 2002;167(4):1715-1719.
- Barbagli G, Kulkarni SB, FossatiN, Larcher A, Sansalone S, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol. 2014;192:808– 813. doi: 10.1016/j.juro.2014.02.038
- Glukhov V.P. Resection of the urethra with end anastomosis for complicated structures and obliterations of the urethra in men: Dis. ...cand. honey. Sci. Saint Petersburg; 2010. Available from: https://www.dslib.net/urologia/rezekcija-uretry-s-koncevym-anastomozom-pri-oslozhnennyh-strukturah-i-obliteracijah.html Link active as of 05/23/2017.
- Heyns CF, Steenkamp JW, De Kock ML, Whitaker P. Treatment of male urethral strictures: Is repeated dilation or internal urethrotomy. J Urol. 1998;160(2):356-8.
- Santucci R, Eisenberg L. Urethrotomy has a much lower success rate than previously reported. J Urol. 2010;183(5):1859-1862. DOI: 10.1016/j. juro.2010.01.020
- Andrich DE, Mundy AR. Urethral strictures and their surgical treatment. BJU Int. 2000;86(5):571-580.
- Kogan M.I., Mitusov V.V., Krasulin V.V., Shangichev A.V., Glukhov V.P. etc. Internal optical urethrotomy for stricture urethral disease complicates subsequent reconstructive surgery. Urology. 2012;3:27-30.
- Naudé AM, Heyns CF. What is the place of internal urethrotomy in the treatment of urethral stricture disease? Nat Clin Pract Urol.2005;2(11):538-545. doi: 10.1038/ncpuro0320
- Mandhani A, Chaudhury H, Kapoor R, Srivastava A, Dubey D, Kumar A. Can the outcome of internal urethrotomy for short segment bulbar urethral stricture be predicted? J Urol. 2005;173(5):1595-1597. doi: 10.1097/01. ju.0000154347.24230.f1
- Dogra PN, Ansari MS, Gupta NP, Tandon S. Holmium laser core-through urethrotomy for traumatic obliterative strictures of urethra: Initial experience. Urology. 2004;64(2):232-235. doi: 10.1016/j.urology.2004.03.050
- Dogra PN, Nabi G. Nd-YAG laser core-through urethrotomy in obliterative posttraumatic urethral strictures in children. Pediatr Surg Int. 2003;19(9-10):652-655. doi: 10.1007/s00383-003-0991-8
- Schneider T, Sperling H, Lummen G, Rübben H. Sachse internal urethrotomy. Is erectile dysfunction a possible complication? . Urologe A. 2001;40(1):38-41.
- Hampson LA, McAninch JW, Breyer BN. Male urethral strictures and their management. Nat Rev Urol.2014;11:43–50. doi: 10.1038/nrurol.2013.275
- Greenwell TJ, Castle C, Andrich DE, MacDonald JT, Nicol DL, Mundy AR. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective ective. J Urol. 2004;172(1):275-277. doi: 10.1097/01.ju.0000132156.76403.8f
- Rourke KF, Jordan GH. Primary urethral reconstruction: The cost minimized approach to the bulbous urethral stricture. J Urol. 2005;173(4):1206-1210. doi: 10.1097/01.ju.0000154971.05286.81
- Buckley JC, Heyns C, Gilling P, Carney J. SIU/ICUD consultation on urethral strictures: Dilation, internal urethrotomy, and stenting of male anterior urethral strictures. Urology. 2014;83(3):S18-22. doi: 10.1016/j. urology.2013.08.075
- Matsuoka K, Inoue M, Iida S, Tomiyasu K, Noda S. Endoscopic antegrade laser incision in the treatment of urethral stricture. Urology. 2002;60(6):968-972.
- Kamp S, Knoll T, Osman MM, Köhrmann KU, Michel MS, Alken P. Low-power holmium:YAG laser urethrotomy for treatment of urethral strictures: Functional outcome and quality of life. J Endourol. 2006;20(1):38-41. doi: 10.1089/end.2006.20.38
- Xiao J, Wu B, Chen LW, et al. Holmium laser urethrotomy for male urethral stricture. Zhonghua Nan Ke Xue. 2008;14(8):734-736.
- Gürdal M, Tekin A, Yücebaş E, Kireççi S, Sengör F. Contact neodymium: YAG laser ablation of recurrent urethral strictures using a side-fi ring fi ber. J Endourol. 2003;17(9):791- 794. doi: 10.1089/089277903770802399
- Kamal B.A. The use of the diode laser for treating urethral strictures. BJU Int. 2001;87(9):831-833.
- Milroy EJ, Chapple CR, Cooper JE, Eldin A, Wallsten H, et al. A new treatment for urethral strictures. Lancet. 1988;1(8600):1424-1427.
- Hebert PW. The treatment of urethral stricture: Transurethral injection of triamcinolone. J Urol. 1972;108(5):745-747.
- Harriss DR, Beckingham IJ, Lemberger RJ, Lawrence WT. Long-term results of intermittent low-friction self-catheterization in patients with recurrent urethral strictures. Br J Urol. 1994;74(6):790-792.
- Kjaergaard B, Walter S, Bartholin J, Andersen JT, Nøhr S, et al. Prevention of urethral stricture recurrence using clean intermittent self-catheterization. Br J Urol. 1994;73(6):692-695.
- Lauritzen M, Greis G, Sandberg A, Wedren H, Ojdeby G, Henningsohn L. Intermittent self-dilatation ateer internal urethrotomy for primary urethral strictures: A case-control study. Scand J Urol Nephrol. 2009;43(3):220-5. doi: 10.1080/00365590902835593
- Pitkamaki KK, Tammela TL, Kontturi MJ. Recurrence of urethral stricture and late results ateer optical urethrotomy: Comparison of strictures caused by toxic latex catheters and other causes. Scand J Urol Nephrol. 1992;26(4):327-331.
- Mundy AR. Adjuncts to visual internal urethrotomy to reduce the recurrence rate of anterior urethral strictures. Eur Urol. 2007;51(6):1467-8. doi: 10.1016/j.eururo.2007.02.061
The article was published in the journal “Bulletin of Urology” No. 2 2021, pp. 69-76
Magazine
Bulletin of Urology No. 2 2017
Comments
To post comments you must log in or register
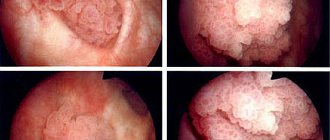
Diagnosis of urethral stricture
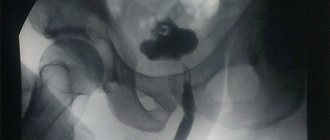
To confirm the diagnosis of urethral stricture, the urologist only needs to perform uroflowmetry, which will demonstrate a decrease in the volumetric flow rate of urination, and urethrography. The last study consists of an x-ray of the urethra, previously filled with contrast.
It is urethrography that makes it possible to determine the extent and degree of narrowing of the urethra, on the basis of which the urologist chooses one or another version of the operation - urethroplasty. Additional information is provided by ultrasound of the bladder and kidneys (sometimes urethral stricture is complicated by the formation of urinary stones).
Materials for urethral stricture surgery
For a long time, penile skin was almost the only material for reconstructive operations (lack of hair, close localization to the surgical area). However, since the early 1990s, buccal mucosal grafts have become increasingly popular. In recent years, there has been increasing interest in the lingual mucosa due to the ease of graft extraction and low incidence of complications. The possibility of using fibrin glue to more securely fix the graft and reduce the frequency of urine extravasation is being considered. Advances in tissue engineering allow us to hope in the near future to solve the issue of donor material shortage, and the introduction of endoscopic urethroplasty may become a new milestone in reconstructive surgery of urethral stricture.
Treatment of urethral stricture. Plastic surgery (surgery) for urethral strictures.
Before starting treatment, the doctor and the patient must clearly understand what its goal is - complete relief of the stricture or temporary relief (which in some cases is also acceptable, but the patient must be clearly aware of this!)
If diagnosis usually does not cause difficulties, then treatment of urethral stricture can be quite a difficult task! Let's start with the fact that conservative (medicinal) treatment of urethral stricture is absolutely ineffective!
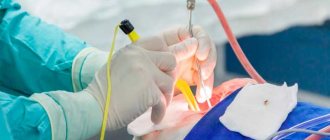
The previously used bougienage of the urethra (expansion of its lumen using special instruments - bougies) is now considered useless. The only option left is surgical treatment. It would seem that it could be simpler - cut the narrowed section of the urethra, install a temporary frame, and wait for healing!
A similar idea occurred to the great Ambroise Pare (Leonardo da Vinci from surgery!) more than 450 years ago, in 1561 he performed an internal urethrotomy with an instrument specially designed for this purpose! Over the past centuries, the equipment of the urologist has changed dramatically, high-quality endoscopic video systems and laser technology for cutting strictures have appeared.
However, the main problem remains - the dissected (but not excised) scar tissue tends to grow back together, through the formation of a new scar! Vicious circle? Exactly!!!
Therefore, urethrotomy as a minimally invasive operation for urethral stricture has the right to exist ONLY for short (up to 5 mm) narrowings in the bulbous region. In other cases, it is useless (and sometimes harmful!).
Repeated urethrotomies, as a rule, lead to the progression of the scarring process both in the urethra itself and in the surrounding tissues.
Classification of urethral strictures
Urethral stricture is best classified as follows:
- By type of disease:
- Short;
- Untreated;
- Simple.
- According to the complexity of the disease:
- Extended;
- Postoperative;
- Double.
- In combination with other diseases:
- After undergoing prostatectomy;
- Based on unfavorable conditions due to the presence of hair, abscess, stones, stent, diverticulum and fistula.
If we consider the formation of strictures from the point of view of pathogenesis, then a special role in both inflammation and injury is played by the negative effect of urine itself on the tissue, because this leads to urinary infections where the filtration function is impaired. Any damage is a sharp shock, which ultimately leads to pathology.
What remains?
The solution has been found! Complete excision of the scarred segment of the urethra provides a GUARANTEE of getting rid of the stricture! For short urethral strictures up to 10-15 mm in length, after excision of the scar area, the ends of the urethra are sutured to each other (anastomotic urethroplasty or Holtsov operation).
Surgical treatment of extended strictures inevitably leads to the need to replace the excised segment of the urethra. For this purpose, many materials have been proposed, both the body’s own tissues and artificial implants. Experience has shown that the best results of urethroplasty are achieved when using the mucous membrane (inner surface) of the cheek or lip.
Thus, plastic surgery for extended urethral strictures using the buccal mucosa (or replacement buccal urethroplasty) is a complex microsurgical intervention with elements of tissue transplantation, the effectiveness of which directly depends on the experience and qualifications of the operating urologist.
I trained in microsurgery and surgical treatment of urethral strictures both in Germany and with the best Russian urethral surgeons, so I am ready to help you treat even complex and extended strictures.
Author: candidate of medical sciences, doctor of the highest category, Rotov Anton Evgenievich
Treatment
It is almost impossible to solve this problem without surgery. In turn, the correct choice of treatment method for urethral stricture in men can only be made by a truly experienced doctor who understands the patient’s needs and knows all the modern methods of treating urethral narrowing. These are the specialists who conduct appointments in our men’s and women’s health clinic. Make an appointment with a urologist who will relieve you of this unpleasant disease quickly, effectively and without unnecessary expenses. The price for visiting our specialists is average in Moscow, but the quality does not suffer from this.
Our clinic's urological surgeons were educated at the best universities and studied with renowned experts. In addition, they have extensive experience in treating urethral strictures. As you know, experience is critical for surgeons.
Depending on the size of the stricture, the doctor may opt for either an endoscopic method or surgery to repair the urethral stricture. This type of operation is especially effective when the stricture is located in the membranous urethra. The methods used to carry it out are also different. All modern methods of surgical treatment of urethral narrowing are effective and safe, the likelihood of relapse is quite low.
The Men's and Women's Health Clinic provides quality medical care at all levels, from diagnosis to post-operative recovery.
| Code | Name | Price |
| 03.73 | Urethroscopy, resection of urethral stricture | 100,000 rub. |
| 03.00 | Initial appointment with a urologist (candidate of medical sciences) | 2,000 rub. |
| 03.04 | Repeated appointment with a urologist (candidate of medical sciences) | 1,200 rub. |
| 03.60 | Initial appointment with a urologist (MD) | 5,000 rub. |
| 03.61 | Repeated appointment with a urologist (MD) | 3,000 rub. |
| All prices of the men's and women's health clinic | ||
Questions about the article
Alexander
May 23, 2021 at 11:27 pm
Good afternoon. I am 31 years old. Help me please. The situation is quite stupid - after sexual intercourse, due to my suspiciousness, I decided to rinse the urethra with an antiseptic with 60% alcohol. I've done this before, there were no consequences. But this time there was a strong burning sensation and after 20 minutes it stopped and so far there seem to be no other symptoms, but nevertheless I am very afraid for my health. Tell me, please, could a stricture occur in this case? Does it occur immediately or after some time?
Anton Evgenievich Rotov
May 25, 2021 at 08:52
A stricture can occur as a consequence of a chemical burn. Since this is a scarring process, it occurs after some time (from 3 weeks to six months). I hope this doesn't happen to you
Lyubov Petrovna
May 6, 2021 at 03:14 pm
Hello! After the round of surgery, my husband had a narrowing of the urethra. On December 9, at the Research Institute of Urology, he underwent surgery to excise the scar. Now urination is difficult again, a urethral catheter was installed until May 11th.
Anton Evgenievich Rotov
May 6, 2021 at 05:42 pm
Urethral stricture, unfortunately, in some cases causes relapses after surgery. To clarify, it is recommended to perform urethrography (a few days after removal of the catheter)
Yuri V.
July 17, 2021 at 11:10 am
I am 56 years old. I have a urethral stricture. I had prostate cancer, I had it removed four years ago, then I had radiation therapy, after that I couldn’t hold urine, they gave me an artificial muscle, a year passed, everything was fine, then the contra ministry began. They resected the canal once and then again a month later. Now they put a catheter in the bladder and said that in life, help me, I am only 56 years old.
drrotov
July 18, 2021 at 02:52 pm
I will be happy to help, for this you will need to come for a consultation with all the available results
Alexey 45
December 15, 2021 at 02:47 pm
Hello. Can you increase the meatus to 2 cm?
drrotov
December 16, 2021 at 17:00
Yes, theoretically this is possible. Ultimately it will depend on the diameter of the urethra. You also need to know for what purpose you want to do this.
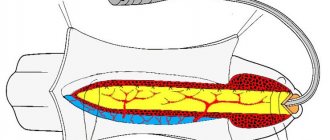
Extent and localization of narrowing
A short narrowing is considered to be up to 2 cm in length, the rest are considered long.
The localization of the narrowing is extremely important; the method of surgical treatment and prognosis depend on this. There are narrowings of the anterior and posterior sections of the urethra. The length of the anterior section reaches 15 cm, the posterior section – up to 4 cm. Damage up to 2/3 of the length is called subtotal (almost complete) narrowing; a larger extent of the stricture is considered total or is called obliteration.
Narrowings of the anterior section include strictures:
- meatus or external opening;
- capitate section;
- scaphoid fossa;
- penile region (pendulous);
- bulbous department.
In the posterior region the following may narrow:
- prostatic;
- membranous.
The exact location and extent of the narrowing is determined during diagnostic procedures.