Date of:
February 22, 2021
Author:
Aleksander Prejbisz, Sylwia Kołodziejczyk‑Kruk, Piotr Dobrowolski, Andrzej Januszewicz
Additional information
For citation:
Prejbisz A., Kołodziejczyk‑Kruk S., Dobrowolski P., Januszewicz A.: Diagnostyka i leczenie hiperaldosteronizmu pierwotnego. Podsumowanie stanowiska European Society of Hypertension 2021. Med. Prakt., 2020; 11:32–40
Abbreviations : AH - arterial hypertension, AMP - mineralocorticoid receptor antagonist, ARP - plasma renin activity, ARS - aldosterone-renin ratio, CT - computed tomography, PHA - primary hyperaldosteronism, AF - atrial fibrillation, DRC (direct renin concentration) - renin concentration , ESH - European Society of Hypertension
Incidence of primary hyperaldosteronism and its clinical picture
The incidence of PHA is estimated to be 3.2–12.7% among primary care patients and 1–30% among specialty care patients. The authors of the ESH position indicate that there is a continuum between the so-called. primary low-renin arterial hypertension (AH) and PHA. PHA is a progressive disease that begins with a phase with normal blood pressure but with decreased plasma renin concentrations and slightly elevated aldosterone concentrations, and gradually progresses to a phase with hypertension and more pronounced hormonal changes.
2. Reasons
Aldosterone is one of the hormones produced by the adrenal glands. Like other hormones, aldosterone is involved in the simultaneous regulation of several physiological processes: in particular, its main functions include maintaining normal blood pressure, acid-base and water-salt balance (the required concentration of sodium and potassium ions). In the strict original meaning of the term, Conn's syndrome is caused by a hormone-producing tumor of the adrenal glands, the secretory activity of which leads to abnormally high aldosterone levels. The work of J. Conn is based on a cause-and-effect relationship between the removal of such a benign tumor (aldosteroma), on the one hand, and, on the other, the subsequent normalization of a number of indicators in operated patients.
Currently, to primary hyperaldosteronism, i.e. Conn's syndrome includes not only those symptom complexes that are caused by benign tumor processes in the adrenal glands. A similar clinical picture can also be given by hyperplasia (i.e. non-oncological growth, enlargement of the adrenal glands in size, which is detected in approximately half of all cases), adrenal cancer (detected in less than 1% of cases), genetically determined hyperproduction of aldosterone by both adrenal glands (also about 1% of all cases of primary aldosteronism).
Visit our Endocrinology page
Who should be suspected of having primary hyperaldosteronism?
Resistant hypertension and grade 3 hypertension: the incidence of PHA increases with increasing severity of hypertension. It is believed that up to 20% of patients with resistant hypertension suffer from PHA.
Hypertension at a young age: Patients at a young age (<40 years) may have milder forms of PHA, and sometimes do not have the classic indications for the diagnosis of PHA. This group of patients may benefit significantly from diagnosis and treatment of PHA in terms of cardiovascular risk and quality of life, although data to support this hypothesis are limited.
Hypokalemia: Numerous studies have assessed the prevalence of hypokalemia in patients with PHA, but the incidence of PHA in patients with hypertension and hypokalemia has not been examined. However, the finding of hypokalemia in a patient with hypertension (without another known cause) should always raise suspicion for PHA. This also applies to diuretic-induced hypokalemia.
Adrenal incidentaloma: PHA is estimated to occur in 1.6% to 4.3% of patients with adrenal incidentaloma (in both those with and without hypertension). In patients with adrenal incidentaloma and hypertension, PGA may be more common.
Family history of primary hyperaldosteronism or early-life stroke: Familial forms of PHA are very rare. Their suspicion may be related to an early stroke in a 1st degree relative or a history of PHA in a 1st degree relative. The familial form of PHA should be suspected only if PHA is detected in the family history of relatives at a young age. Because PHA is relatively common, its occurrence in older relatives may be coincidental and does not necessarily indicate a genetic predisposition.
Diabetes mellitus, metabolic syndrome and obstructive sleep apnea: The ESH position authors indicate that disorders of glucose metabolism, including diabetes mellitus, are more common in patients with PHA than in the general population of patients with hypertension. Regarding obstructive sleep apnea, it was emphasized that the vast majority of patients with obstructive sleep apnea suffering from PHA have one of the indications for the diagnosis of PHA listed in the document.
Atrial fibrillation (AF) is significantly more common in patients with PHA than in the general population of patients with hypertension. Results from a study that included patients with AF not associated with structural heart disease or other disease indicate a relatively high proportion of patients with PHA in this group (42%).4 However, the results of this study should be interpreted with caution because the inclusion criteria patients with AF of unknown etiology raise some doubts - only 18% of patients from the population of patients with AF and hypertension were included in the study, although at the same time the authors concluded that the coexistence of hypertension itself does not explain the occurrence of AF. Unclear inclusion criteria may result in non-representativeness of the population being assessed and difficulty in extrapolating results to the entire population of patients with AF.
Screening for primary aldosteronism
As a screening test in patients with suspected PHA, it is recommended to determine plasma aldosterone and renin concentrations and then calculate the aldosterone-renin ratio (ARR; the ratio of plasma aldosterone to renin concentrations), which is still considered the most reliable and accessible screening method.
Existing differences in methods for determining renin concentration (DRC), plasma renin activity (PRA), and aldosterone do not allow establishing a single cut-off point for ARS; In the case of aldosterone concentration expressed in ng/dL and ARP in ng/ml/h, the most commonly used cut-off point for APC is 30 (but values in the range 20–40 are also acceptable) - see table. Therefore, individual laboratories and institutions develop their own standards. Table. Cut-off points for the aldosterone-renin ratio (ARR) as indications for further diagnosis depending on the method of measurement and the accepted units of determination of renin and plasma aldosterone concentration
| ARP (ng/ml/h) | DRC (mIU/l) | |
| Aldosterone concentration ( ng / dl ) | 20 | 1,3 |
| 30 | 2 | |
| 40 | 2,7 | |
| Aldosterone concentration ( pmol / l ) | 550 | 36 |
| 830 | 55 | |
| 1100 | 74 | |
| ARP – plasma renin activity, DRC – renin concentration based on ESH1 position | ||
The concentration of aldosterone and renin in plasma is best determined after discontinuation of the drugs affecting them. If necessary, the patient can take verapamil, doxazosin and moxonidine. If drugs that affect aldosterone and renin levels (β-blockers, dihydropyridine calcium channel blockers, diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists [sartans]) cannot be discontinued, the ARS should be assessed and the result interpreted taking into account the effect of the drugs being taken patient. This test should not be performed in patients taking an aldosterone antagonist. Before determining aldosterone and renin concentrations, hypokalemia should be corrected without restricting sodium intake. Blood samples should be collected before noon from the patient in a sitting position, after at least 2 hours of standing upright (at which time the patient can sit but cannot lie down).
The center presented by the authors of this article uses 30 as the cutoff point for APC for aldosterone and renin concentrations expressed in pg/mL. If laboratory values are expressed in other units, they can be converted to pg/mL by multiplying the aldosterone concentration in ng/dL by 10 and dividing the renin concentration in mIU/L by 1.67. It should also be noted that if the APC is in the range of 20–30, the clinical picture is analyzed and on this basis some patients may be selected for further diagnosis of PHA.
Figure 1 shows examples of interpretation of the results of determining APC. Interpretation should consider whether aldosterone concentrations are elevated. The question is not whether it exceeds the upper limit of normal, that is, the reference interval adopted in a given laboratory. Aldosterone concentrations are suggested to be >150 pg/mL (15 ng/dL), although some authors recommend a cutoff point of 100 pg/mL or the cutoff value for diagnosing PHA in a confirmatory test (i.e., >100 pg/mL or >60 pg/ml in sodium loading test). The renin concentration should be low, that is, below or close to the lower limit of the reference range.
Picture 1. Aldosterone-renin ratio (ARR) is a screening assessment for primary hyperaldosteronism (PHA). An example of a ratio interpretation based on the 2021 ESH position and the authors' own experience of this article.
Studies confirming the diagnosis of primary hyperaldosteronism
A positive ARS result mandates one of the recognized studies supporting PHA, but the ESH position authors suggest that in patients with idiopathic hypokalemia, plasma renin concentrations below the detection threshold or very low and resting aldosterone concentrations >200 pg/mL (20 ng/dL) PHA can be diagnosed without the need for a confirmatory study.
The ESH position lists the 4 most commonly used confirmatory tests: the oral and intravenous sodium loading test, the captopril test, and the fludrocortisone test, emphasizing that the choice of test should depend on the availability of the test and the experience of the particular institution. As with the ARS determination, medications that affect the renin-angiotensin-aldosterone system should be taken into account and contraindications to the test should be assessed - in the case of a sodium load test, these are: significantly elevated blood pressure, severe heart failure and renal failure.
The ESH position authors discussed the possibility of recommending a single test and cutoff values for diagnosing PHA. Due to the lack of sufficient data, no decision on specific values was made. Figure 2 suggests a diagnostic algorithm for PHA, developed on the basis of the discussed position and experience of the authors of this article. In practice, the most commonly used test today is the seated sodium load test. The most commonly used cutoff to diagnose PHA in this setting is an aldosterone concentration >60 pg/mL (170 nmol/L) with a cortisol concentration lower after a 0.9% NaCl infusion than initially determined. Threshold values for the supine sodium load test are also accepted: >100 pg/ml (definite diagnosis) and 50–100 pg/ml (probable diagnosis). The ESH position states that a sodium loading test performed in a sitting position may be the preferred method, and if it is contraindicated, a captopril test may be performed.
Figure -2. Algorithm for diagnostic tactics in people with suspected primary hyperaldosteronism. The given limit values for individual diagnostic stages were proposed based on the position of ESH and the experience of the authors of this article. They should be considered as examples and take into account the methodology used and the experience of the institution
Differentiation of forms of primary hyperaldosteronism
CT scan
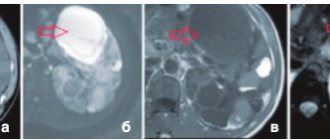
All patients with PGA should have a contrast-enhanced computed tomography (CT) scan of the abdomen to rule out adrenal cancer and assess venous drainage before adrenal vein catheterization, and in patients selected for surgery, an anatomical assessment before adrenalectomy.
The authors of the ESH position emphasize that the agreement of CT findings with those obtained from adrenal venous catheterization is not satisfactory. The largest meta-analysis to date, including 38 studies and a total of 950 patients with PHA, found that the imaging study (CT or MRI) was different from the adrenal venous catheterization result in almost 38% of cases. It should be noted that anatomical imaging of the adrenal glands cannot determine the diagnosis of PHA and differentiate its form. The presence of bilateral adrenal hyperplasia on imaging does not indicate the presence of PHA. It is necessary to conduct hormonal tests. Also, the detection of an adenoma in a patient with PHA (on CT) does not determine the diagnosis of a unilateral form (with the exception of the specific clinical picture described below).
Catheterization of the adrenal veins
Determining the form of PHA is key when choosing a treatment method. In all patients with unilateral disease who are appropriately selected for surgery, adrenalectomy normalizes serum potassium levels, produces normotension in 30–60% of patients without the need for antihypertensive medications, and improves blood pressure control in the remaining patients. On the other hand, patients with bilateral PHA do not benefit as much from adrenalectomy and should be treated pharmacologically.
The ESH position continues to emphasize the importance of adrenal vein catheterization as the “gold standard” in determining the form of PHA. The authors discuss in detail the principles of performing this procedure and interpreting the results - we recommend that those interested consult this document.2
Young patients (<35 years) with idiopathic hypokalemia, significantly increased aldosterone secretion (plasma aldosterone concentration >300 pg/mL), a typical unilateral adenoma (>10 mm) on CT, and a normal morphologic appearance of the second adrenal gland may not require adrenal catheterization. veins before adrenalectomy.
4.Treatment
Obviously, the therapeutic strategy in each case is determined by the diagnosed causes of hyperaldosteronism. Thus, for unilateral aldosteroma, the method of choice is laparoscopic adrenalectomy; The effectiveness of surgical intervention in this case is very high - in almost all patients, blood pressure and other aldosterone-related indicators gradually normalize.
In other cases, conservative treatment may be preferable, the main goal of which is to prevent severe complications caused by hypertension and hypokalemia. Special potassium-sparing drugs, antihypertensive drugs, ACE inhibitors, and a special diet are prescribed. In some cases, hormonal therapy (dexamethasone, hydrocortisone) is indicated and effective. Necessary measures are also the normalization of body weight and therapeutic exercises in the air.
Treatment of primary hyperaldosteronism
Patients with PHA are characterized by an increased risk of cardiovascular complications compared with patients with idiopathic hypertension, which is associated not only with the long-term effects of elevated blood pressure, but also with the damaging effects of excess aldosterone itself. Both adrenalectomy and drug therapy with mineralocorticoid receptor antagonists (MRAs) reduce this risk.
The ESH position recommends performing unilateral laparoscopic adrenalectomy in people with documented unilateral disease. Other patients should receive ongoing pharmacological treatment with MRA. This treatment should also be added in patients with a positive screening test (APS) who do not agree to proceed with or are contraindicated for diagnostic testing.
In patients with bilateral PHA, the treatment of choice is spironolactone, and in case of intolerance, eplerenone. The dose of the medicine should be gradually increased. It is believed that the optimal dose of MRA should provide normokalemia without the need for potassium replacement. It is also believed that a reduction in the incidence of cardiovascular complications in PHA is achieved only in the presence of complete blockade of mineralocorticoid receptors, as evidenced by non-reduced renin concentrations.5 If the dose used is uncertain, the renin concentration should be measured - if it is still low, this means that complete blockade of receptors has not occurred and the dose should be increased.
The use of eplerenone is limited by the lack of registration for use in hypertension and PHA in Europe. It should be remembered that eplerenone is used in a dose approximately 2 times higher than the dose of spironolactone and in 2 divided doses.
Hyperaldosteronism
A syndrome caused by hyperproduction of aldosterone by the zona glomerulosa of the adrenal cortex, accompanied by arterial hypertension and myasthenia.
The cause of hyperaldosteronism
can be a hormonally active adenoma of the adrenal cortex (aldosteroma), bilateral hyperplasia of the zona glomerulosa of the adrenal cortex, multiple microadenomas of the adrenal cortex.
Hyperaldosteronism can develop with chronic kidney disease, hypertension, and some kidney tumors. The cause of hyperaldosteronism can also be long-term use of medications: diuretics, laxatives, contraceptives. A transient state of hyperaldosteronism is observed during the luteal phase of the menstrual cycle, during pregnancy, and with sodium restriction in the diet. Depending on the cause, clinical practice varies:
1. Aldosteronism with low renin secretion
- Primary aldosteronism as a result of a tumor of the glomerular layer of the adrenal cortex (Conn's syndrome).
- Idiopathic hyperaldosteronism is diffuse hyperplasia of the adrenal cortex.
- Dexamethasone hyperaldosteronism suppressed by glucocorticoids.
- Hyperaldosteronism caused by ectopic tumors.
2. Aldosteronism with normal or increased secretion of renin (secondary hyperaldosteronism).
- Symptomatic arterial hypertension in renovascular pathology, kidney disease, hypertension.
- Renin-secreting tumors (Wilms tumor).
- Iatrogenic and physiological hyperaldosteronism.
Pathogenesis of the disease
associated with excess secretion of aldosterone.
The main action of aldosterone is to regulate sodium reabsorption and potassium excretion in the renal tubules. With hyperaldosteronism, retention of sodium ions and potassium excretion develops, which is accompanied by hyperkaliuria, hypokalemia, and hypernatremia. As a result of hypokalemia, hypokalemic nephropathy occurs, which is accompanied by polyuria, dystrophic changes in the muscles and myocardium. The development of hypertension is caused by sodium retention, its accumulation in the walls of the arteries, their swelling, increased tone and sensitivity to pressor agents. In the presence of an adrenal adenoma, aldosterone synthesis is autonomous. Clinical picture.
The initial symptom is early hypertension.
Patients complain of headaches and aching pain in the heart area. The stage of severe symptoms is characterized by blurred vision, muscle weakness, cramps, and muscle pain. Renal symptoms are pronounced: polyuria, isohyposthenuria, nocturia, secondary polydipsia. From the cardiovascular system: cardiac arrhythmia, tachycardia, systolic and diastolic arterial hypertension. Diagnostic criteria:
- Combination of hypertension and myasthenic syndrome.
- Hypernatremia, hypokalemia, hyperkaliuria.
- Polyuria, iso- and hyposthenuria. Urine reaction is alkaline.
- Increased plasma aldosterone levels and its excretion in urine.
- Increased size of the adrenal glands on ultrasound, CT or angiography.
- Signs of hypokalemia on the ECG.
To clarify the diagnosis
functional tests are carried out, tests with veroshpiron, with a load of sodium chloride, furosemide.
Treatment
depends on the causes of hyperaldosteronism. For primary hyperaldosteronism, surgical treatment is indicated. For secondary hyperaldosteronism, long-term drug treatment is carried out with spironolactone, potassium preparations, and glucocorticoid synthesis inhibitors. Regardless of the etiology of the disease, the diet should contain a limited amount of table salt and foods rich in potassium (potatoes, dried apricots, rice, raisins).