ARTIFICIAL KIDNEY
- a device for removing toxic metabolic products and exogenous poisons from the body, as well as for regulating electrolyte-water balance and acid-base balance through dialysis and blood ultrafiltration.
An artificial kidney temporarily replaces the functions of the kidneys to maintain homeostasis (see), but does not simulate renal processes (glomerular filtration, tubular reabsorption and secretion, etc.) and endocrine function.
Hemodialysis (see) is the release of blood from crystalloids due to the selective diffusion of substances through a semi-permeable membrane. In the Artificial Kidney, this membrane separates the sterile blood system from the non-sterile system that carries the dialysate. Depending on the permeability of the membrane, its area, the design of the apparatus, the temperature of the solution, the difference in the concentrations of substances on both sides of the membrane, the size and shape of their molecules, etc., dialysis of different substances in different types of I. p. does not occur equally quickly.
Ultrafiltration (see) - removal of water from the body due to the difference in hydrostatic and osmotic pressure on both sides of a semi-permeable membrane. The pressure gradient necessary for ultrafiltration is achieved in I. p. Ch. arr. due to positive pressure in the blood supply system and negative pressure in the dialysate solution system. The process of water removal can be enhanced by increasing the osmotic pressure of the dialysate solution by adding osmotically active substances (glucose, mannitol).
Story
Work on the creation of IP began with the research of Abel (JJ Abel) et al. (1913), who developed the first prototype of hemodialysis equipment, called the “artificial kidney”. The apparatus consisted of a series of tubes made of a semi-permeable membrane (collodia), placed in a glass cylinder and combined into a common manifold at the beginning and end of the cylinder. The cylinder was filled with physiol. table salt solution (dialysis solution). The device was connected to the animal's blood vessels, allowing blood to circulate through it using blood pressure. To prevent blood clotting, the anticoagulant hirudin was administered. Studies have shown that metabolic products and toxic substances of exogenous origin can be removed from the blood. These studies were of great scientific importance, but the equipment and methods of its use were very imperfect.
Necheles (H. Necheles, 1923) was the first to use a device he created, in which the peritoneum was used as a semi-permeable membrane to remove products of protein metabolism in dogs with experimental uremia. The first use of I. p. in humans was carried out by Haas (G. Haas, 1925-1927). The device he created was used to treat uremic intoxication. Despite the absence of an obvious wedge effect, it was possible to note the removal of a certain amount of nitrogen metabolism metabolites with the solution. In Haas's work, a special pump was used for the first time to move blood through the apparatus. To create artificial hemophilia during hemodialysis, Lim (R.K.S. Lim) and Neheles (1926) first used heparin (see) instead of the previously used toxic and difficult to standardize hirudin.
For a long time, a serious difficulty in creating I. p. was the lack of a semi-permeable membrane that meets the requirements of hemodialysis. Numerous variants of such membranes (collodion, fish swim bladder, calf peritoneum, etc.) due to their non-standard nature and low mechanical strength turned out to be unacceptable for widespread use. This problem was solved by W. Thalhimer (1938), who was the first to propose and test cellophane for this purpose.
The accumulated experience, especially the use of heparin and cellophane, created the necessary prerequisites for the development of the first variant of the I. p. apparatus, suitable for wide wedge use, created in 1943 by physician W. Kolff in collaboration with engineer N. Berk. Soon N. Alwall (1946) created IP, which, along with dialysis, could provide ultrafiltration. In subsequent years, treatment with I. p. began to be used on an increasingly wider scale. A large number of models were created, differing in the design of a number of main components.
In our country, the creation of the IP apparatus began in 1955 on the initiative of Academician. V.V. Larina. For the first time, the I. p. apparatus was used for the treatment of patients with renal failure in early 1958 by A. Ya. Pytel, N. A. Lopatkin, and the first Soviet device, created by a group of doctors and engineers (M. G. Ananyev, Yu. G. Kozlov, E. B. Gorbovitsky and others), - in 1960.
Hemodialysis machines
If kidney function is impaired, dialysis is used today, when a special machine is used to cleanse the blood of waste, salts and excess fluid. But this procedure, usually performed in a hospital, usually three times a week, is very tiring for patients and only works when a person is connected to this machine in a clinic or dialysis center. Today, several teams of scientists are working on this problem to make life easier for such patients.
System One
NxStage Medical has created the System One dialysis system, which can be used directly in patients' homes without the involvement of medical professionals. It is the only device in the world that is FDA-approved for overnight hemodialysis, allowing you to receive treatment as usual during the day or at night while you sleep. In this case, the device can use both dialysate in standard containers and the PureFlow SL system, which prepares dialysate from tap water. System One is a lightweight device, which allows you to move it to any place convenient for the user or even take it with you when traveling.
AWAK PD
AWAK Technologies has developed a wearable peritoneal dialysis system, AWAK Peritoneal Dialysis (AWAK PD). Peritoneal dialysis purifies the blood using the patient's peritoneum as a membrane filter. The AWAK system allows dialysis on the go, eliminating the problem of long treatment hours and the need to connect to a dialysis machine.
In this type of dialysis, the peritoneal dialysis solution is administered through a permanent tube into the abdominal cavity, and the purification process takes place there. The fluid remains in the abdominal cavity for a certain period of time before it is drained and disposed of. If this method is compared with hemodialysis, then its advantage is that it can be used by young children.
This world's first such device has significantly changed the way peritoneal dialysis is performed.
Essential elements
All I. p. devices, despite the variety of design solutions, have the same basic design and consist of the following main elements: 1) dialyzer; 2) a perfusion device to move blood through the device; 3) a device for preparing and supplying dialysing solution to the dialyzer; 4) devices that monitor and regulate the basic technical and medical parameters of hemodialysis (monitor).
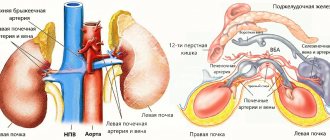
Rice. 1-4. Schematic illustrations of various dialyzers. Rice. 1. Coil dialyzer: 1 - blood input; 2 — dialyzer body; 3—output for dialysate solution; 4 - drainage fittings; 5 - spirally coiled tubular membrane; 6 — input for dialysate solution; 7 - output for blood. Rice. 2. Capillary dialyzer: 1 - blood inlet; 2 - distribution chamber; 3 — dialyzer body; 4 — input for dialysate solution; 5—output for blood; 6—capillaries; 7 — output for dialysate solution; 8 - sealing layer. Rice. 3. Disposable plate dialyzer: 1 - external pressure plates of the housing; 2—dialyzer plate; 3 - membranes; 4 - distribution board; 5—body clamps; 6 — input for dialysate solution; 7—output for blood; 8 — output for dialysate solution; 9—inlet for blood. Rice. 4. Plate dialyzer SGD-6: 1 — upper clamping frame; 2— dialyzer plate; 3—membrane; 4 — input for dialysate solution; 5 — output for blood; 6 - blood distributor; 7—rubber plate seal; 8 — coupling bolts; 9—distributor for dialysate solution; 10 — lower clamping frame; 11—inlet for blood; 12 — output for dialysate solution; Bottom left—general view of the dialyzer.
Dialyzer (hemodialyzer)
. A significant number of different variants of dialyzers have been developed and are used, differing in design, materials and membranes used (color fig. 1-4). All of them can be divided into main groups: dialyzers in the form of drums (movable and fixed); in the form of coils; plate type dialyzers; capillary.
The first I. p., developed by W. Kolff, had a dialyzer in the form of a rotating drum, on which a cellophane tube was wound spirally in one layer. The blood in the tube moved according to the principle of an Archimedes screw. Although the Kolff dialyzer and dialyzers with a fixed drum (Alvalla, Battesatti, Meller) are practically not used due to their bulkiness, complexity of assembly, large volume of primary filling and other disadvantages, they were the basis for subsequent developments.
Coil-type dialyzers, developed by W. Kolff and W. Watchinger in 1956, are a tube of semi-permeable membrane wound in several layers on a solid cylindrical base. To prevent an increase in the volume of the tube when blood circulates through it, it is limited on both sides by plastic mesh, which at the same time does not impede the contact between the membrane and the dialysate solution.
Plate-type dialyzers were first developed by Skeggs and Leonards (L. T. Skeggs, JR Leonards, 1948). In these dialyzers, the main structural elements are sheets of semi-permeable membrane, which are fixed between polymer plates equipped with longitudinal grooves, with the help of which a directed flow is formed when the dialyzer is filled with blood. From the outside, the semi-permeable membrane is washed with dialysate solution. By combining the required number of the above-mentioned structural elements, it is possible to obtain dialyzers with a given working area. Plate dialyzers include models Kiil, Gambro, Rhone - Poulenc, as well as dialyzers used in domestic IP machines (AIP-140, Diachron-80, SGD-6, Diacentre-1, etc.).
The so-called are widely used. capillary dialyzers. They are based on thin-walled (11-30 microns) capillaries made of a semi-permeable membrane with an internal diameter of about 200-260 microns. Collected into bundles containing thousands of such tubes, they are placed in cylindrical cases made of transparent plastic. At the beginning and end of such a cylinder, all spaces between the capillaries are sealed with a special compound, thus separating. blood supply system from the circulation system of the dialysate solution entering through the side fittings of the cylinder.
Semi-permeable membranes used in I. p. are the most important functional element of the apparatus. The effectiveness of I. p. and its safety for the patient mainly depend on their characteristics. There are the following basic requirements for the membrane, it must:
1) not have an adverse effect on the blood and do not release toxic products upon contact with it (the semi-permeable membrane accounts for at least 95% of the entire foreign surface with which the blood comes into contact when passing through the I. p.); 2) ensure effective removal of metabolites and toxic products of exogenous origin; 3) provide the required ultrafiltration rate; 4) do not skip protein; 5) have high strength, preventing membrane rupture under mechanical loads and temperature conditions.
Most of the membranes used are made from cellulose derivatives (cellophane, cuprofane, nephrophane, etc.). The pore sizes in these membranes are 1.5–2.5 nm, and the thickness of the membranes ranges from 10 to 20 μm. Membranes differ significantly in dialyzing activity and ultrafiltration ability, which allows for their rational selection during hemodialysis.
A certain place in the development of uremic syndrome is given to the so-called. medium molecular metabolites, chemical. the nature of which has not been deciphered. In this regard, membranes have been developed (based on polyacrylic nitrile and some other polymers) that are characterized by significantly higher rates of purification of medium molecular substances compared to conventional membranes for hemodialysis. These membranes have several times greater ultrafiltration activity, which makes it possible to remove larger amounts of fluid during hemodialysis (up to several liters per hour), and also use them in special ultrafiltration units (for example, to sharply reduce the volume of ascitic fluid before its reinfusion). However, in most cases, the excessively high ultrafiltration activity of these membranes creates difficulties associated with the need to carefully monitor fluid losses during hemodialysis and promptly compensate for excess fluid losses. Research is being carried out aimed at creating membranes that would have a high cleaning ability in relation to medium molecular metabolites and at the same time maintain ultrafiltration activity close to the activity of membranes made from cuprofane, etc. At the same time, membranes with high ultrafiltration rates may be indispensable for the new development type of apparatus I. p., the action of which is based on the principle of diafiltration.
Dialyzers are available with different dialysis surface areas (from 0.24 to 2.5 m2) in accordance with the characteristics of their application (for example, with a small area for pediatric practice, etc.). Depending on this, the cleaning and ultrafiltration power, as well as other parameters (for example, the volume of primary filling) change. The most important parameters characterizing various dialyzers are dimensions, sterilization method, preparation time for use, primary filling volume, residual volume, clearance of low molecular weight substances, clearance of medium molecular substances, ultrafiltration, internal resistance, membrane rupture rate, reusability .
Dialyzers are available for both single and multiple use. All capillary and coil dialyzers and a number of plate dialyzer models are single-use. These dialyzers are supplied fully assembled, sterilized and ready for immediate use. Some of them can be reused if certain precautions are taken. According to the European Association of Dialysis and Transplantation (1975), when hemodialysis is carried out in a hospital, single-use dialyzers are more often used; when treating at home, both types are used with approximately equal frequency.
The main indicators of the effectiveness of I. p. are clearance and dialysis, which show how much dialyzed fluid is completely cleared of a given substance per unit of time (min.) at the selected perfusion rate. Clearance and dialysis are calculated using Wolf's formula (A. Wolf, 1952):
C = (A - R)*a/A; D = (A - R)*a/(A - U),
where C is clearance (ml/min); D—dialysis (ml/min); A is the concentration of the substance at the entrance to the dialyzer; R is the concentration of the substance at the outlet of the dialyzer; U is the concentration of the substance in the dialysate solution; a — speed of dialyzed fluid (ml/min).
Clearance characterizes the device with constant updating of the solution, in which there is no test substance at the entrance to the dialyzer. Dialysis also characterizes the device during recirculation of the dialysate solution, when during the dialysis process the concentration of the test substance in it increases and the clearance decreases accordingly.
To determine the effectiveness of I. p., the clearance of urea and creatinine is usually examined. For this purpose, urea solutions (3 g/l) and creatinine solutions (0.2 g/l) are used. One study requires approx. 20 l solution. Dist is used as a dialysing solution. water. The temperature of both solutions is 37°. The study is carried out while the dialyzed solution passes through the dialyzer at a speed of 50 to 250 ml/min. Samples are taken after the dialyzer has been operating under stable conditions for approx. 15 minutes.
Perfusion device.
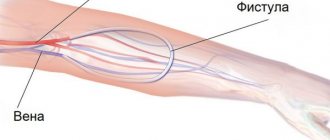
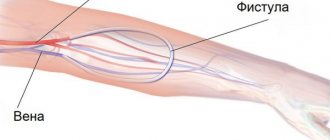
The patient can be connected to the device in various ways. In acute cases, when there is hope for a rapid restoration of kidney function, special catheters inserted into large veins are often used. In case of hron, renal failure or prolonged course of acute renal failure, arteriovenous shunts or arteriovenous fistula are most often used (see Hemodialysis). When using the catheterization method and when working with an arteriovenous fistula, it is necessary to use special perfusion pumps, while an arteriovenous shunt often provides blood flow in a device without a pump. There are different types of pumps: diaphragm, roller, sigma pumps. Roller pumps are most often used. Sometimes blood flow meters (Doppler, electromagnetic, ball, etc.) are also used to assess the intensity of perfusion. To introduce various drugs into the blood, in particular heparin and protamine sulfate, two types of pumps are usually used: syringe pumps and, less commonly, roller pumps.
Device for preparing and supplying dialysate solution.
Experience with the use of i.p. has shown that the dialysing solution must contain cations of sodium, potassium, calcium, magnesium and anions of acetate (or bicarbonate, lactate) and chlorine. In case of severe extravascular hyperhydration, one or another amount of glucose is sometimes added to the solution, but not more than 3000 mg%, since higher concentrations can lead to complications (hyperglycemia, convulsions, etc.).
The dialysis solution can vary within certain limits, for example, depending on the wedge, requirements, as well as on the composition of the water used to prepare the solution. The potassium content exhibits the greatest variations. The amount of calcium and magnesium introduced into the solution may depend on their content in the water. In some places, tap water contains an excess of calcium, magnesium and other salts, and then preliminary water preparation is required, for which several methods are used. Distillation allows you to obtain well-purified water, but this method is expensive, so demineralization of water using ion exchange resins is more often used.
In a simpler version, when only calcium and magnesium need to be removed, water softening is used, which is achieved using only cation exchangers. The method of water preparation using reverse osmosis has increasingly become part of the practice of hemodialysis. For mechanical purification of water from rust, organic suspensions and other contaminants, various filtration systems are used.
Dialysis solution is prepared in two ways. In a number of I. p. devices, dry salts or their concentrated solutions are added to a certain volume of water in order to obtain the desired concentration after they are dissolved. In addition, they use an automatic method for the continuous preparation of dialysate solution from the so-called. concentrate and water. In the concentrate, the content of all its constituent salts is 30-35 times higher than in the dialysate solution. Therefore, one part of the concentrate is diluted with 34 parts of water, which is done using special devices - dispensers.
Dialysis solution in I. p. is used in three ways. 1. Recirculation. The finished dialysate solution is in one container and, after passing through the dialyzer, is returned to the same container. Since the concentration of metabolic products washed out of the body in the solution is constantly increasing, it requires periodic replacement with fresh solution. The frequency of the change is determined by the capacity of the tank and the rate of accumulation of slag in the solution. 2. “On-drain” system. The dialysate solution passes through the dialyzer once and is discharged into the sewer.
There are systems in which the spent solution is subjected to regeneration, after which it enters circulation again. In fact, the principle of recirculation is used here, but the nature of dialysis is closer to a “drain” system. 3. Combination of recirculation and drain system. A certain amount of dialysate solution flows through the dialyzer at high speed, but a certain amount of used solution is constantly drained into the sewer system and at the same time the same amount of fresh solution is added to the tank.
Monitoring the operation of the artificial kidney. Chem. control over the composition of the dialysate solution is carried out by flame photometry (determining the concentration of sodium, potassium ions, etc.) - If we are talking about a recirculation system, then one determination at the beginning of dialysis is sufficient.
In systems operating “on the drain”, the composition of the dialysate solution is constantly monitored using salinity meters. Typically two instruments are used to increase the reliability of the observation. The work of salt meters, in turn, is also periodically checked using flame photometry (see) or osmometry (see Osmotic pressure).
In addition to chem. control, during hemodialysis requires constant monitoring of the temperature of the dialysate solution. All hemodialysis systems are equipped with heaters and thermostats that automatically maintain the set temperature of the solution.
When working with a bicarbonate buffer, to stabilize the pH of the dialysate solution, it is purged with a mixture of oxygen and carbon dioxide, and therefore frequent monitoring of the pH of the solution is required.
Modern I. p. devices include monitors that provide monitoring of blood pressure in the device, the entry of air into the patient’s bloodstream, blood leakage into the dialysing solution, as well as the speed of passage of the dialysing solution through the I. p. and its pressure in the dialyzer . Measuring the amount of ultrafiltration during hemodialysis is carried out by Ch. arr. using a scale bed that is not included in the I. p. If the integrity of the membrane is damaged and blood leaks into the dialysis solution, automatic systems for stopping hemodialysis are triggered. The so-called level detectors, or air detectors, which monitor the blood level in a special chamber (air trap) of the venous line, which reliably prevents the possibility of air embolism. Pressure sensors monitor the level of positive blood pressure in the dialyzer. The speed of movement of the solution can also vary widely, and therefore almost all I. p. devices are equipped with flow meters (flow meters).
The safety of the patient is based on the fact that the I. p. has devices that, in the event of an increase or decrease in the specified conditions, give off light and sound alarms. In some cases, such as, for example, when the set blood pressure is exceeded, when air enters the blood supply system, blood enters the dialysis solution, or when the temperature of the dialysis solution increases above the established limits, the blood flow and dialysis are automatically interrupted. Interruption of blood flow is carried out using electromagnetic clamps.
Kidney Project (University of California, San Francisco)
The most famous development in this area is carried out within the framework of the Kidney Project, which employs specialists from the University of California, San Francisco and Vanderbilt University (USA). They have developed an implant the size of a coffee cup that can perform the functions of a kidney and is a great solution for patients with chronic kidney disease. This small, surgically implanted device consists of a highly permeable filtration block and human kidney cells.
The filter component has micropores that can be individually shaped to perform specific tasks. These filters can be placed one after the other, each of which will perform a different filtering function. In total, the device contains fifteen such filters, placed one on top of the other. In between and around these filters are living kidney cells that perform functions that artificial components do not do well, including reabsorbing nutrients and getting rid of accumulated waste. Since such a hybrid biological device would be placed out of reach of the body's immune response, it would thus be protected from rejection by the human body.
Those. The device contains living cells and could theoretically not only filter blood, but also perform other important functions of a real kidney, such as releasing hormones to control blood pressure.
In addition to filters that separate various substances in the blood, the implant includes a “bioreactor” that processes the ultrafiltrate, extracts sugar and salts from it, which it returns to the blood. In this process, water is also reabsorbed back into the body, and the ultrafiltrate is converted into “urine”, which is sent to the bladder for removal from the body. These devices are housed in a durable housing, covered with a film of material that is safe for use inside our body. The implant is connected by tubes to nearby veins and the bladder.
The device is powered by the patient's blood pressure and does not require the use of external tubing or cables that are now associated with wearable artificial kidneys.
Purified blood is returned to the circulatory system through veins connected to the implant, and waste is transferred to the bladder through a corresponding tube. All elements through which blood passes are coated, which should prevent the formation of blood clots. In addition, the formation of blood clots is prevented by a specially designed blood flow pattern within the system.
According to the developers, the device will not perform all the functions of a human kidney. “But the goal is for it to perform critical functions and be a system that, once implanted, allows the patient to eat and drink freely, have mobility, better overall health, and, unlike a transplant, not require the use of immunosuppressive drugs,” say university scientists.
Note that patients must still take the hormonal supplements they use during dialysis procedures.
Testing of a working prototype is expected to take place in 2020.
Sterilization
Sterility and pyrogen-free dialyzers and blood circulation systems are more reliable in disposable devices. All elements that come into contact with the patient’s blood during dialysis are collected in the factory, packaged and sterilized using ethylene oxide, gamma irradiation or formaldehyde. A significant disadvantage of single-use systems is their high cost. Therefore, reusable dialyzers are still widely used. In these cases, preparation of the device for operation is carried out directly in the hospital. institution. Before use, the dialyzer and blood lines are filled with 2% formalin solution, which gives the most complete sterilization effect. Immediately before the operation, the system is washed with sterile physiol solution with heparin (2 liters of physiol solution are required). Preparing the device for operation takes only 30-40 minutes. The dialysate system is periodically sterilized with formaldehyde. This is especially important for recirculating devices, since in this case bacterial contamination of the dialysis system occurs very quickly, which can lead to pyrogenic reactions due to the penetration of bacterial toxins through the membrane.
Recommendations
- "Kidney Anatomy: Overview, Macro Anatomy, Microscopic Anatomy." 2017-08-29. Magazine citation required | log = (help)
- "Kidneys and how they work." www.niddk.nih.gov
. Received 2015-11-30. - "Kidney Review". WebMD
. Retrieved 2015-12-02. - "Key Points: About Dialysis for Kidney Failure." www.kidney.org
. National Kidney Foundation. 2021 - ^ a b c d f f
Johnson, Stephen (2014-10-11).
“Demand for dialysis is increasing as kidney disease rises.” www.modernhealthcare.com
. Modern healthcare. - Bywaters EGL, Beall D (1941). "Diseases with impaired renal function." British Medical Journal
.
1
(4185): 427–32. doi:10.1136/bmj.1.4185.427. PMC 2161734. PMID 20783577. - ^ a b c
"Fast Facts".
National Kidney Foundation
. National Kidney Foundation. 2014-08-12. Retrieved 2016-11-13 - via kidney.org. - Twardowski, Zbylut J. (August 9, 1994). "Artificial kidney for frequent (daily) hemodialysis." US Patent
. - Fissell W. H., Humes HD, Fleischman A. J., Roy S. (2007). “Dialysis and nanotechnology: now, 10 years or never?” Blood purification
.
25
(1): 12–17. Doi:10.1159/000096391. PMID 17170531. - Lindsay RM, Leitch R, Heidenham AP, Kortas C (2003). "The London Daily/Nocturnal Hemodialysis Study: Study Design, Morbidity and Mortality Results." Am J Kidney Dis
. 42 Appendix 1: S5 – S12. Doi:10.1016/S0272-6386(03)00531-6. - Fissell W, Manley S, Westover A, Humes HD, Fleischman AJ, Roy S (2006). "Differentiated growth of human renal tubular cells on thin-film and nanostructured materials." ASAIO Journal
.
52
(3):221–227. Doi:10.1097/01.mat.0000205228.30516.9c. PMID 16760708. - “The artificial kidney implantation project is progressing successfully - News and problems of nephrology.” News and problems of nephrology
. 2016-02-25. Retrieved 2016-12-07. - "World's first implantable artificial kidney could enter human trials by 2021." Medical Device Online
. Retrieved 2016-12-07. - ^ a b c d f g gram hour i j
Ronco, Claudio;
Davenport, Andrew; Gura, Victor (07/01/2008). "Towards a wearable artificial kidney." International hemodialysis
.
12
: S40 – S47. Doi:10.1111/j.1542-4758.2008.00295.x. ISSN 1542-4758. PMID 18638240. - ^ a b
Gura, Victor;
Rivara, Matthew B.; Bieber, Scott; Munshi, Raj; Smith, Nancy Kolobong; Linke, Laurie; Kundzins, John; Beizai, Masoud; Ezon, Carlos (2016). "Wearable artificial kidney for patients with end-stage renal failure." JCI Insight
.
18
). doi:10.1172/jci.insight.86397. ISSN 2379-3708. PMC 4936831. PMID 27398407. - ^ a b
Gura, Victor;
Macy, Alexandra S.; Beizai, Masoud; Ezon, Carlos; Golper, Thomas A. (12/07/2016). "Technical Breakthrough in Wearable Artificial Kidney (WAK)." Clinical Journal of the American Society of Nephrology
.
4
(9):1441–1448. Doi:10.2215/CJN.02790409. ISSN 1555-9041. PMC 2736696. PMID 19696219. - ^ a b
Markovich, M.;
Rapin, M.; Correvon, M.; Perriard, Y. (09/01/2013). "Design and optimization of a perfusion pump for a wearable artificial kidney device." IEEE Transactions on Industry Applications
.
49
(5):2053–2060. doi:10.1109/TIA.2013.2260851. ISSN 0093-9994. - ^ a b
Kim, Jeong Chol;
Garzotto, Francesco; Nalesso, Federico; Cruz, Deanna; Kim, Ji Hyun; Kang, Eungtaek; Kim, Hee Chan; Ronco, Claudio (2011). "Wearable artificial kidney: technical requirements and possible solutions." Examination of medical devices
.
8
(5):567–579. Doi:10.1586/erd.11.33. PMID 22026622. - Saito A., Aung T., Sekiguchi K., Sato Y, Wu D., Inagaki M., Kanai G., Tanaka R., Suzuki N., Kakuta T. (2006). “Current status and prospects of bioartificial kidneys.” J Artificial Organs
.
9
(3): 130–5. Doi:10.1007/s10047-006-0336-1. PMID 16998696. - Saito A, Aung T, Sekiguchi K, Sato Y (2006). "Current status and prospects for the development of a bioartificial kidney for patients with chronic renal failure." Ther Apher Dial
.
10
(4): 342–7. Doi:10.1111/j.1744-9987.2006.00387.x. PMID 16911187. - Wang P., Takezawa T. (2005). "Reconstruction of renal glomerular tissue using collagen vitrigel". J Biosci Bioeng
.
99
(6):529–40. doi:10.1263/jbb.99.529. PMID 16233828. - Fissell W, Fleischman AJ, Roy S, Humes HD (2007). "Development of continuous renal implantation: past and future." Translational Research
.
150
(6):327–336. doi:10.1016/j.trsl.2007.06.001. PMID 18022594. - «Home | Kidney Project | UCSF." Pharm.ucsf.edu
. Retrieved 2018-12-11. - “Artificial kidneys eliminate dialysis.” 2017-11-09.
Possible complications and side effects
The blood purification procedure is not as safe as we would like. An artificial kidney in some cases leads to side effects such as:
- itching of the skin at the site of catheter insertion;
- decreased red blood cell levels;
- pressure surges;
- pain and muscle spasms;
- sleep disorders;
- decrease in bone strength.
There is also the possibility of more serious complications, such as inflammation of pericarditis. Equipment failure may occur, leading to the death of the patient.
Function of artificial kidney
The “artificial kidney” device is used if the patient’s own organ loses its functionality by 85-90%. This device helps:
- remove urea from the blood;
- improve metabolic processes;
- remove excess fluid;
- control acid-base balance;
- prevent the formation of blood clots.
In addition, it helps to saturate the blood with air, which improves the patient’s well-being. Thanks to modern portable technology, hemodialysis can be performed at any convenient time, without leaving home.