Neuroprotectors in the treatment of glaucoma - review of drugs
Neuroprotectors have been used in the treatment of stage glaucoma not so long ago. At the same time, the drugs protect the retina and optic nerve. This type of therapy is aimed at correcting metabolic disorders, improving microcirculation, tissue nutrition, normalizing the rheological properties of blood, and establishing basal and lateral circulation.
It should be noted that this technique is effective only when the level of intraocular pressure is reduced through drug therapy, laser and surgical treatment.
Classification
There are four degrees of changes in nerve fibers in glaucoma:
- Irretrievably lost;
- Acute phase of degeneration;
- Dystrophic changes;
- Preserved structure.
Neuroprotectors are divided into two groups:
- Direct directly protect the neurons and fibers of the retina and optic nerve, respectively.
- Indirect neuroprotectors increase the body's resistance to a decrease in reperfusion pressure.
The selection of specific antiglaucoma therapy requires the doctor to systematically examine the patient. It is carried out on the basis of hemodynamic disorders and metabolic changes. The effectiveness of treatment should be monitored every six months. Below are the main groups of neuroprotectors.
Calcium channel blockers
Drugs in this group increase cell resistance to ischemic effects and also dilate blood vessels. The most commonly used is betaxolol. This medicine reduces vascular resistance and increases neuronal stability. Due to good permeability, the active substance quickly penetrates the structures of the eye and acts on the receptors already in the first hour after instillation.
To reduce the level of pressure inside the eye, betaxolol is instilled twice a day, but sometimes the frequency is increased to 3-4 times.
The use of this medication is contraindicated in patients with cardiac dysfunction and rhythm, corneal dystrophy, and hypersensitivity. Patients with diabetes mellitus, thyrotoxicosis, muscle weakness, and Raynaud's syndrome should be careful. The same applies to pregnant women. Before planned general anesthesia, it is advisable to discontinue the drug.
During therapy, it is necessary to monitor the condition of the eyes (production of tear fluid, integrity of the epithelium) at least once every six months.
When betaxolol is used topically, the development of systemic side effects is unlikely.
Preparations that contain betaxolol as an active ingredient:
- Betoptik (0.5% solution);
- Beoptic S (0.25% solution).
Enzyme antioxidants
Superoxide dismutase is one of the body's natural antioxidant protectors. It destroys reactive oxygen species and has an anti-inflammatory effect. Due to this, the development of degradation in the structures of the trabecular meshwork and optic nerve fibers is inhibited.
Already 1-2 hours after instillation, the maximum concentration of the drug in the tissues of the eye is determined. It penetrates the choroid and retina, accumulating in them.
The drug is prescribed 5-6 times a day. Sometimes they use the method of forced instillation, when the drug is instilled every 10 minutes for an hour. The course of treatment is 2 months.
Drugs produced by various manufacturers:
- Erisod. It is a lyophilized powder (400 thousand and 1.6 million units), from which eye drops are prepared.
- Rexod (800 thousand units).
Non-enzymatic antioxidants
Histochrome can neutralize iron ions, which usually accumulate in the ischemic area.
It also neutralizes free radicals, improves energy metabolism and normalizes the rheological properties of blood. The maximum concentration of the drug is reached an hour after administration.
Routes of drug administration include subconjunctival and prebulbar. The duration of the course of therapy is 10 injections.
The drug Histochrome is available in the form of a 0.02% solution in ampoules.
Succinic acid has a positive effect on metabolic processes. At the same time, the ionic permeability of the membrane decreases, calcium metabolism is regulated, etc. salts of this acid are components of many dietary supplements (mitomin, yantavit, enerlit).
Succinate-containing heterocyclic compounds (for example, Mexidol) are more promising drugs. This drug forms a buffer redox system. It has a positive effect on energy processes in cells, activates the synthesis of nucleic acids, and enhances glycolysis. Mexidol improves blood flow in the ischemic zone and promotes rapid healing of defects.
Mexidol should not be prescribed for hypersensitivity or in cases of serious liver and kidney diseases. The most common side effects are dyspepsia, dry mouth and allergies.
Mexidol is administered intramuscularly (100 mg) twice a day. The course of therapy is 10-14 days. The drug is available in the form of a 5% solution.
Emoxipine is one of the oldest drugs for the treatment of eye diseases accompanied by ischemia. This substance is a structural analogue of vitamin B6. The drug stabilizes the red blood cell membrane and plays an important role in microcirculation disorders. The maximum concentration is observed after 15-30 minutes, during which the substance accumulates in the retinal cells.
When treating with emoxipine, monitoring of the blood coagulogram is required. Do not mix the medicine in the same syringe with other drugs. The effectiveness of treatment increases if alpha-tocopherol is taken orally at the same time.
Emokipine can be administered by instillation, periocular injection, or as an ocular film. The frequency of instillations is usually 5-6 times per day. The course of treatment lasts 2-4 weeks.
The drug is available in the form of a 1% solution or eye films.
Neuropeptides
Cytomedins are alkaline polypeptides. By acid extraction they are purified from impurities. These substances stimulate cell differentiation processes, affect humoral and cellular immunity, hemostasis, and microcirculation.
Cytomedins, which are obtained from brain and retinal tissues, are involved in the regulation of nervous tissue. Nowadays, cortexin and retinalamin are used in ophthalmology.
Retinalamin is administered intramuscularly, parabulbarly (once a day), Cortexin is administered only intramuscularly. The course of therapy lasts 10 days.
To improve hemodynamics, you can use angioprotectors and antispasmodics.
Antispasmodics
Purine and indole alkaloids are used in clinical practice. They increase the concentration of cAMP in the vascular wall and inhibit platelet aggregation.
Theophylline (250 mg three times a day) or xanthinol nicotinate (150 mg three times a day) is usually prescribed.
Indole alkaloids include vinpocetine (taken orally 5 mg three times a day). To increase efficiency, the course can be started with intravenous administration.
Purine alkaloids include chirantyl, trental. They improve the rheological properties of blood when used daily.
Angioprotectors
These drugs normalize microcirculation, vascular permeability, eliminate tissue edema associated with impaired permeability of the vascular wall, reduce the activity of plasma kinins and stimulate metabolic processes. In practice, doxium, parmidine, and etamsylate are used.
Vitamins and nootropics help correct metabolic disorders.
Nootropics
Most often, piracetam is prescribed from this group of drugs, which improves microcirculation, metabolic processes and increases glucose utilization. The use of the drug is contraindicated in cases of severe renal failure, hemorrhagic stroke, or hypersensitivity.
The medication will be prescribed orally at 30-160 mg/kg/day. The course of therapy is 6-8 weeks.
Also in the doctor’s arsenal there are combination products containing piracetam and cinnarizine. Prescribe the medicine 1-2 capsules three times a day. The course of therapy is 1-3 months.
Gamma-aminobutyric acid derivatives (picamelon) are also used. It has a vasodilator and nootropic effect. Another analogue of GABA is nooclerin.
The drug Semax is an analogue of ACTH. It improves energy metabolism in neurons, increases their resistance to hypoxia and damage. It is instilled into the nose, from where it is absorbed into the systemic bloodstream through the vessels of the mucous membrane. The duration of treatment is 5-14 days. The drug is also used for endonasal electrophoresis (Semax is administered from the anode).
Source: https://glaucomacentr.ru/vse-o-glaukome/344-nejroprotektory-pri-glaukome
Vision restoration - independent vision restoration without surgery
Veselovskaya Z.F., Veselovskaya N.N. Primary neuroprotection in glaucoma ZF Veselovskaya, NN Veselovskaya
Kiev City Ophthalmological Center Department of Ophthalmology of Kiev State Medical University, Kiev Purpose: to study the effectiveness of calcium channel blocker (CCB) agents in glaucoma treatment. Materials and methods: Patients with early glaucoma participated in the trial. Ophthalmologic examination consisted of testing of visual acuity, perimetry, tonometry, pachymetry, OCT. Also biochemical blood assay was taken, and observation was also carried out by cardiologists, neurologist and internal medicine specialist. In the main group amlodipine was prescribed by 5 mg per day, betaxolol ophthalmic solution and travoprost ophthalmic solution. In control group travoprost was used as a hypotensive agent. Observation lasted 3 years. Results: 63 patients with newly diagnosed POAG were taken into 1 sample, 43 — in to the second one. In both groups IOP level decreased by 20-25% from the baseline. All examined indicators were stable in the main group during all observation period. Worsening of all functional indicators without visual loss was registered in the 2nd group. Conclusion: CCB agents have obvious neuroprotective effect and could be recommended for use in complex treatment of patients with glaucoma.
Relevance An analysis of modern statistics on visual impairment due to glaucoma indicates that in the last 20 years, about 27% have lost vision in one eye, and about 9% of patients with glaucoma . Even the use of antihypertensive therapy does not fundamentally affect the situation as a whole, since bilateral blindness during treatment is recorded in 4.4% of patients with glaucoma . Thus, normalization of IOP is not a guarantee of achieving a stable level of visual function. This situation cannot but alarm ophthalmologists around the world. Today they are joining forces to achieve consensus on the pathogenesis, diagnosis and treatment of glaucoma . Multilateral research is being actively conducted to find optimal ways to solve this serious problem. At the same time, according to the recommendations of the European Glaucoma Society and international standards, it is envisaged to maintain a fairly high quality of life for patients with glaucoma for as long as possible. At present, there is no doubt that the progression of optical neuropathy in glaucoma is associated with vascular dysregulation at the local and systemic level, rheological and other metabolic disorders, which result in chronic ischemia and hypoxia. Neurophysiological studies have shown that excessive release of glutamate under ischemic conditions has a neurotoxic effect due to the uncontrolled entry of calcium ions into the nerve cell through the calcium ion channels of its somatic membrane. The neurodystrophic nature of optical glaucomatous neuropathy necessitates the search for effective pharmacological drugs with neuroprotective properties. Assessing the effectiveness of neuroprotective treatment presents certain difficulties, since its results can only be seen after a sufficiently long period of time. Neurophysiological research in recent years has made it possible to identify pharmacological drugs that have direct and indirect neuroprotective properties. It has been established that the direct neuroprotective effect is realized only at the level of the neural synapse, i.e. somatic membranes of the nerve cells included in it. Today it has been proven that calcium channel blockers (CCBs) and NMDA receptor blockers (NMDA) have this effect. Thus, CCBs (Betoptik S, norvasc, amlodipine, etc.) block calcium channels of the presynaptic membrane, prevent the excessive entry of calcium ions into the axon terminal and the excessive release of the neurotransmitter glutamate into the synaptic cleft. BNMDA (mammothin, etc.) bind glutamate-chemoregulated ion channels of the postsynaptic membrane, preventing excessive entry of calcium ions into the nerve cell. Drugs of the BCC group are widely known today in cardiological and neurological practice. The pronounced effect of CCBs is due to the fact that direct neuroprotective properties (blockade of calcium channels in the somatic membrane of nerve cells) are also enhanced by vasoselective or indirect neuroprotective properties through blockade of calcium channels in the somatic membrane of smooth muscle cells of the vascular wall. The results of our joint project with the Institute of Physiology named after. A.A. Bogomolets NAS of Ukraine (1999-2007) proved that BCCs have not only neuroprotective, but also pronounced neuroretinoprotective properties due to the regulation of the flow of Ca2+ ions through high-threshold calcium ion channels of the somatic membrane of ganglion cells and axon terminals, creating a barrier to the development of neurotoxic damage to ganglion cells in conditions of chronic ischemia [5,6,13,14]. Purpose To study the effectiveness of pharmacological drugs from the group of calcium channel blockers in the complex treatment of patients with glaucoma. Materials and methods A total of 63 patients (33 with POAG and 30 with IGT) were monitored for 3 years (regular examination once a quarter). Control group II consisted of 43 patients (23 with POAG and 20 with IGT). The study groups included only patients with newly diagnosed glaucoma. The ophthalmological examination included determination of visual acuity (VA), visual field (FO) by computer campiperimetry (CCP), intraocular pressure (IOP) by non-contact tonometry (BT) and according to Maklakov, central corneal thickness (CCT) by contact keratopachymetry, thickness of the nerve layer fibers (TSNV) using optical coherence tomography (OCT). The systemic examination included a biochemical blood test (coagulogram, lipidogram), observation by a cardiologist, neurologist and therapist. Complex treatment of patients in the main group included constant intake of Norvasc or amlodipine (5 mg per day) in agreement with a cardiologist and Betoptik S eye drops. Patients in the control group did not receive drugs from the CCB group. Travatan was prescribed to compensate for IOP in both groups. Twice a year, patients in both groups received a course of vascular therapy (Actovegin, Mildronate, vitamin C, Milgama or Neurovitan). Results A comparative analysis of the initial distribution of patients by age, gender, presence of concomitant diseases (HD), as well as the results of determining VA, IOP, PV, TSNV, TCR indicated the quantitative and qualitative identity of the composition of the main and control groups. IOP control showed that patients in both groups achieved a reduction in IOP by 20-25% from the initial level. A comparative analysis of the dynamics of changes in the studied indicators over 3 years based on the results of digital research methods indicated a stable level of stabilization of all studied indicators in the main group. In the control group, an irreversible deterioration in morphofunctional indicators was recorded without a decrease in central visual acuity. Thus, a decrease in TSNV by 5-10% (according to OCT data) and retinal photosensitivity with the MD coefficient (according to CCP data) by 10-15% was noted in 7 patients with POAG (30.4% of cases) and in 9 patients with IGT ( 44.1% of cases). Thus, despite strict adherence to the medication regimen, in the control group, 30.4% of patients with POAG and 44.1% with IGT experienced a gradual deterioration in morphofunctional parameters, indicating the progression of glaucomatous optic neuropathy. This was more true for patients with normal-tension glaucoma. The stability of morphofunctional parameters in patients with both POAG and normal pressure glaucoma was noted in patients of the main group. It can be assumed that the prescribed course of therapy with the inclusion of drugs from the CCB group for both systemic and local use created a certain level of constant neuroretinoprotection or protection of ganglion and other cells at the level of the visual analyzer by maintaining the physiological level of calcium homeostasis, at the level of the somatic membrane of the ganglion cells. and other cells of the neural synapse. Without direct neuroretinoprotection at the systemic and local levels, this level of protection cannot be ensured, as evidenced by the negative dynamics of morphofunctional indicators in the control group.
Conclusions 1. Modern data on the role of ischemia in the development of optic neuropathy in glaucoma provide justification for the use of drugs with direct neuroprotective action in the complex treatment of glaucoma. 2. Calcium channel blockers, by controlling the entry of calcium ions into the ganglion cell and vascular endothelium, have a pronounced neuroretinoprotective effect by enhancing the direct neuroprotective effect with a concomitant vasoselective, or indirect, neuroretinoprotective effect. 3. Systemic and local use of CCBs in the complex treatment of patients with glaucoma creates conditions for long-term protection of retinal ganglion cells at the level of their somatic membrane from the destructive effects of ischemia, ensuring a more stable balance in the morphofunctional state of the visual analyzer in patients with glaucoma.
Literature 1. Anisimova S.Yu. Neuroprotective therapy for glaucoma // Biologist. honey. - 2002. - P. 39-42. 2. Anisimov S.I. Results of conservative treatment of partial atrophy of the optic nerve in glaucoma // Glaucoma: All-Russian. scientific-practical Conf.: Materials. - M., 1999. - P. 332-333. 3. Astakhov Yu.S. Neuroprotective effect of the drug with retinols in the treatment of primary open-angle glaucoma using endonasal electrophoresis // ROOF: Coll. scientific work. - M., 2010. - T. 1. - P. 232-236. 4. Bunin A.Ya. Metabolic factors in the pathogenesis of primary open-angle glaucoma // Glaucoma at the turn of the millennium: results and prospects: All-Russian. scientific-practical Conf.: Materials. - 1999. - pp. 9-12. 5. Veselovska Z.F. A new approach in drug therapy for primary low-grip glaucoma based on calcium channel blockers // Ophthalmol. magazine - 2006. - No. 3. -T. 1. - pp. 88-89. 6. Veselovskaya Z.F. Modern aspects of neuroprotection in the treatment of chronic vascular pathology of the visual analyzer // Problems of environmental and medical genetics and clinical immunology: Zb. Sci. fuck off. - Lugansk, 2011. - pp. 80-86. 7. Vesnina N.A. Complex treatment of glaucoma // Glaucoma: theories, trends, technologies: VIII international. Conf.: Sat. scientific stat. - M., 2010. - P. 83-84. 8. Volkov V.V. An essential element of the glaucomatous process, not taken into account in clinical practice. Ophthalmol. magazine - 1976. - No. 7. -S. 500-504. 9. Zavgorodnyaya N.G. Primary glaucoma. A new look at an old problem // Zaporozhye. - 2010. - 184 p. 10. Nesterov A.P. Primary open-angle glaucoma: pathogenesis and principles of treatment // Klin. ophthalmology. - 2000. - T. 1. - No. 1. - P. 4-5. 11. Levi A. An introduction to neuroprotection in glaucoma/ mechanism and implication // Europ. J.Ophthalmol. - 1999. - Vol. 9. - P. 7-8. 12. Levin LA Neuroprotection for glaucoma // Ethis Communications Inc. - New York, 2007. - 88 p. 13. Rogawski MA Low-affinity channel blocking (uncompetitive) NMDA receptor antagonists as therapeutic agents-toward an understanding of their favorable tolerability // Amino Acids. - 2000. - Vol. 19. - P. 133-149. 14. Weinreb RN Glaucoma neuroprotection // Wolters Kluwer Health. - Philadelphia, 2006.- 114 p.
Share on social media networks
RќСЂР°РІРёС‚СЃСЏ
Glaucoma drops
The main goal of glaucoma treatment is to reduce intraocular pressure to prevent damage and preserve optic nerve function.
As a rule, treatment of glaucoma begins with the prescription of drops that reduce intraocular pressure. These drops must be dripped constantly, strictly according to the doctor’s instructions.
The regularity of their instillation is, to a certain extent, a guarantee of the success of treatment. If some drops are ineffective in maintaining normal intraocular pressure, the doctor may strengthen your regimen by prescribing additional drops of another chemical group, especially since some drops can enhance the effect of others.
All eye drops are absorbed through the superficial vessels into the ocular bloodstream and, albeit in small quantities, still enter the systemic circulation. The active chemicals in some glaucoma drops have undesirable side effects on the body's cardiovascular and respiratory systems, so it is very important for your ophthalmologist to know your common chronic diseases.
If the patient, due to his general condition or some other reason, is not able to regularly instill glaucoma drops, then it is better to offer him other treatment methods.
Types of drops to reduce intraocular pressure
Today, there are many different drugs for the treatment of glaucoma in the form of eye drops, which are classified:
- by active chemical substance: prostaglandins, beta blockers, cholinomimetics, etc.,
- by the mechanism of reducing intraocular pressure: reducing the production of intraocular fluid, improving the outflow of intraocular fluid and drugs with a combined mechanism of action.
Prostaglandin analogues
Prostaglandins are highly effective and safe drugs for glaucoma. Intraocular pressure decreases 2 hours after instillation, the maximum effect is achieved after 12 hours.
These drugs: Travatan (Alcon), Xalatan (Pfizer), Tafluprost, etc., appeared relatively recently. However, due to their high efficiency and duration of action (they need to be instilled only once a day), they have established themselves as the drug of choice in the treatment of glaucoma.
Their mechanism of action is based on improving the outflow of intraocular fluid through an additional outflow pathway.
But prostaglandin group drugs have a number of side effects: transient redness of the eyes (due to dilated superficial vessels), changes in the color of the iris (it becomes darker) and increased eyelash growth (they become thicker, longer and darker).
The latest side effect of prostaglandins, which pleases some patients, has also found its way into cosmetics companies.
Beta blockers
This group of drugs reduces the production of intraocular fluid. They begin to act 30 minutes after application with maximum effect after 2 hours, so the frequency of instillation is usually 2 times a day. They are often prescribed in combination with prostaglandins to enhance the effect.
Timolol, Arutimol, Okumol, Okupress, Okumed - this is an incomplete list of existing beta blockers used in the treatment of glaucoma. As a rule, they have the same active chemical substance, so they are interchangeable.
But these drugs have a number of side effects: decreased heart rate, bronchospasm, etc. Therefore, these drugs are contraindicated for people with certain heart diseases, bronchial asthma, and emphysema.
There are highly selective beta blockers Betoptik and Betoptik S (Alcon), their side effects on the cardiovascular and respiratory systems are less pronounced than other drugs in this group.
Carbonic anhydrase inhibitors
Carbonic anhydrase inhibitors: Azopt (Alcon), Trusopt, reduce the production of intraocular fluid. These are highly effective and safe drugs that have no side effects on the cardiovascular and respiratory systems, but should be used with caution in people with certain kidney diseases.
Carbonic anhydrase inhibitors are usually given twice a day and can be given in combination with other drops, such as beta blockers or prostaglandins, if necessary.
The tablet drug Diacarb has the same active ingredient as Azopt and can also be used in the treatment of glaucoma, especially in acute and subacute attacks of glaucoma.
Cholinomimetics
These drugs (Pilocarpine, Carbocholine) improve the outflow of intraocular fluid by constricting the pupil and contracting certain groups of intraocular muscles, which leads to easier passage of intraocular fluid through the angle of the anterior chamber.
Pilocarpine, as the main representative of this group, is most often prescribed for narrow-angle or closed-angle glaucoma in order to open the drainage network from the root of the iris. Prescribed on average 1-2 times a day. If necessary, pilocarpine is prescribed in combination with other drugs, for example, beta blockers, carbonic anhydrase inhibitors, prostaglandin analogues.
Side effects of pilocarpine are caused by a narrow pupil, which can limit the field of vision and cause unpleasant pain in the forehead, eyebrows, and temple.
Combination drugs
Drops have been developed containing several active chemicals in one bottle. When prescribing several drugs at the same time, and this happens quite often, the use of combined drops reduces the number of bubbles and sometimes reduces the frequency of instillation.
Such drugs are:
- Xalacom (Pfizer) = Xalatan + Timolol.
- Cosopt (Merck) = Trusopt + Timolol
- Fotil = Pilocarpine + Timolol
Neuroprotectors in the treatment of glaucoma
Neuroprotective treatment, i.e. Treatment aimed at improving nutrition and blood supply to the optic nerve plays an important role in the prognosis and course of glaucoma. Studies examining changes in the optic nerve during glaucoma prove the feasibility and necessity of neuroprotective treatment for any form of glaucoma, especially in advanced and advanced stages.
It is important to note that there are studies proving some neuroprotective effect of antiglaucoma drops: prostaglandin analogues, beta blockers, etc. But this, as a rule, is not enough.
Today, many drugs are known, of different origins and chemical compositions, that have certain neuroprotective properties. Main groups of neuroprotectors:
- Medicines that improve blood circulation: ginkgo biloba, trental, dicinone, etc.
- Agents that improve the regeneration of nervous tissue: picamelon, cinnarizine, nootropil, fezam, etc.
- Agents that activate the nutrition of nervous tissue: retinalamin, cortexin, Semax, Cerebrolysin, Noben, etc.
- Antioxidants and vitamins: emoxipin, mexidol, aevit, rutin, ascorbic acid, vitamin E, B vitamins, riboxin, lutein complex, mertilen forte, histochrome, erisod, etc.
Source: https://www.vseozrenii.ru/glaznye-bolezni/kapli-ot-glaukomy/
Neuroprotection and neuroregeneration in glaucoma
Consequently, ophthalmologists can learn from the experience of their neurologist colleagues and use the drugs that they use in the treatment of chronic neurodegenerative diseases. In this regard, akatinol memantine deserves attention, which, unfortunately, did not show its effectiveness in glaucoma in phase 3 clinical trials in multicenter studies, in contrast to Alzheimer's disease, for which this drug was approved for use by the FDA committee in the United States. Canadian scientists tested memantine on primates. They took a small group and got impressive results - neurons were preserved in all layers of the lateral geniculate bodies and the cerebral cortex (slide 2).
Slide 2. Use of akatinol memantine in primates (an experimental model of glaucoma). Noticeable preservation of neurons of the lateral geniculate body during treatment
The first publication on this topic appeared in 2006. Another important observation was made by N. Gupta and Y. Yucel on a patient, when they observed a 79-year-old woman who suffered from glaucoma during her lifetime. She was followed for 19 months for normal tension glaucoma and was treated with dorzolamide. She died of viral myocarditis. At autopsy, significant reductions in the lateral geniculate body and visual cortex were found, similar to those observed in primates with experimental glaucoma. Research on primates is currently ongoing in Toronto. In 2011–2013, scientists began studying dendrites and found that a decrease in their number is the earliest indicator that a cell is entering a state of apoptosis, as information transfer between cells is disrupted. A 2010 publication on dendritic plasticity in the lateral geniculate body showed that experimental glaucoma in primates is the earliest group of dendrites to decrease in number. Consequently, there are prospects for treating glaucoma at an early stage. These studies are currently ongoing.
In the next part of the report, Prof. N.I. Kurysheva dwelled on the general features of the pathogenesis of glaucoma and Alzheimer's disease, emphasizing that the theory of protein misfolding (i.e., disruption of the correct folding of a protein molecule) is currently considered as leading along with the theory of oxidative stress, excitotoxic phenomenon and autoimmune inflammation. A natural question is: how often does glaucoma occur in Alzheimer's disease? Research results on this matter are very contradictory. In fact, one in four people with Alzheimer's disease has glaucoma. In both diseases, amyloid beta deposition and activation of caspases were detected; in Alzheimer's disease, the EDZN is increased and there is thinning of the retinal nerve fibers, as well as impaired transsynaptic transmission. Moreover, it was found that even genetic factors characteristic of Alzheimer's disease are found in glaucoma.
Is there a real-world treatment for neurodegenerative pathology? The answer, unfortunately, is negative. Apart from akatinol memantine, used for Alzheimer's disease, there is no positive information on this matter yet.
Today, ophthalmologists can only rely on the fact that drugs for local antihypertensive treatment have direct neuroprotective properties.
The first publication on this topic dates back to 1996-1997, when prof. Osborne demonstrated the neuroprotective effect of betaxolol in experiments on rabbits and in a culture of isolated ganglion cells. It turned out that this drug actually increased the survival rate of GCS in experimental glaucoma. It has been shown to reduce calcium entry into the cell, reduce neuronal apoptosis, and very soon a study was conducted comparing betaxolol with timolol and brimonidine.
There have been a lot of publications about the direct neuroprotective properties of brimonidine over the past 10 years, which has practically made this drug number one in the treatment of glaucoma.
These properties of brimonidine are explained by its selective effect on alpha-2 receptors. It is their excitation that leads to a complex effect on retinal neurons, protecting them from apoptosis.
In 2003, Stefano Gandolfi conducted a very interesting experiment on patients in the clinic.
In two groups of patients (one used laser trabeculoplasty, the other was treated with brimonidine), they first determined the rate of progression of glaucoma, and then began treatment and looked at the rate at which progression would continue. They found that laser treatment only slowed progression, and brimonidine improved visual fields. Thus, clinical evidence of the direct neuroprotective effect of brimonidine was obtained. Another study conducted in the United States in normal-tension glaucoma showed that progression was three times less with brimonidine than with timolol, but, unfortunately, there were side effects.
A chapter in one of the latest journals “Progress in Brain Research” is called: “Glaucoma - an open window into neuroprotection, neuroregeneration.” This means that using glaucoma as an example, it is possible to search for treatment for neurodegenerative pathology. Ophthalmologists should collaborate with neurologists, conduct joint studies and symposiums, because the experience may be unique.
The next speaker was Professor Bernhard Sabel, Director of the University of Neurophysiology and Psychology at the University of Magdeburg (Germany). The idea was to invite prof. Sabel was dictated by the fact that in the absence of effective drug neuroprotective treatments, the method developed by prof. Sabel deserves attention. This was evidenced by the reaction of ophthalmologists at the world congress in Tokyo, where all the reports of Prof. Sabel was received with great enthusiasm. Currently prof. Sabel conducts unique research in clinics in China; such famous glaucomatologists of the world as R. Ritch and R. Weinreb refer their patients to him for treatment.
The speaker began by emphasizing the important role of the brain in ensuring visual function and the lack of understanding of this aspect by ophthalmologists in all countries.
The cerebral cortex, which processes visual signals, has 300 times more neurons than the retina.
MRI studies have shown that patients with glaucoma have changes in the frontal lobe of the cerebral cortex compared to the same region of the brain of healthy people of the same age. It was concluded that when visual functions are impaired, not only the visual analyzer is affected, but also other areas of the cerebral cortex.
As a result of experiments on rodents, B. Sabel concluded that by preserving 10-20% of retinal neurons, it is possible to restore up to 80% of visual functions over time, and this is ensured by the functioning of the brain. In glaucoma, gray areas visible during perimetry reflect the functioning of neurons with reduced visual function, but still alive. These are areas of residual vision.
The essence of restoring the functions of these neurons is the synchronization of waves in the cerebral cortex. This can be monitored using electroencephalography. When using a special helmet with sensors, you can see the relationship between the work of different centers in the brain and understand whether they are synchronized or not in their functions (slide 3).
Slide 3. Electroencephalography, which allows us to identify the synchronization of waves in the cerebral cortex and the relationship between the work of different centers in it
If we compare patients with glaucoma and a control group (healthy individuals) and analyze the waves in the cerebral cortex in both groups, it turns out that healthy individuals differ from patients with glaucoma precisely in the nature of alpha-2 waves.
Pages: 2
Neuroprotectors and glaucoma
Drug neuroprotection for glaucoma is prescribed to normalize and activate metabolic processes in the eye. A properly selected therapeutic course will reduce the frequency and severity of attacks of the disease, strengthen the optic nerves and provide the necessary nutrition to the tissues of the cornea.
Neuroprotectors: what are these medications and why are they needed?
The main source of painful symptoms in glaucoma is excessively increased eye pressure and dysfunction of the nerve fibers of the eye. Conservative drug neuroprotective therapy can correct the second factor and partially influence the first.
The use of neuroprotective agents is not practiced as a separate type of treatment, but is used in combination with eye gymnastics, training on simulators, laser and surgical intervention to minimize the negative impact of the disease.
With the help of properly selected medications, you can strengthen the neural fibers of the optic nerves, which will have a positive effect on visual acuity, and also balance intraocular pressure, preventing its increase due to the outflow of excess fluid.
Types of medications
For this purpose, patients are also prescribed injectable forms of drugs.
For conservative drug therapy for glaucoma, a combination of pharmaceuticals is used that neutralizes negative symptoms: relieve swelling, remove excess intraocular fluid, stimulate metabolic processes and blood microcirculation, strengthen neuronal fibers and nourish corneal cells, preventing dystrophy.
According to the publication in the RMJ “Clinical Ophthalmology” No. 2, 2014, neuroprotection is one of the most promising strategies for the treatment of glaucoma. Ophthalmologists prescribe tablet medications and injections - intramuscular, intravenous and ocular, as well as instillation and instillation of eye solutions. Complex therapy includes:
- antispasmodics;
- nootropics;
- angioprotectors;
- neuropeptides;
- calcium channel blockers;
- fermented and non-fermented antioxidants.
Neuroprotectors do not treat glaucoma, but are used to relieve painful symptoms.
Beta blockers in the treatment of glaucoma. Back to the Future (literature review)
The authors analyze the safety and effectiveness of topical beta-adrenergic drugs for the treatment of glaucoma. Particular attention is paid to prolonged dosage forms of timolol maleate. Key words: glaucoma, beta-blockers, timolol maleate, Timolol gel. Abstract Beta-blockers in the treatment of glaucoma. Back to the future. Literature review Egorov EA, Egorov AE
RNRMU named after IN Pirogov Authors analyze the effectiveness and safety of topical beta-adrenergic drugs for treatment of glaucoma. Particular attention is paid to prolonged formof timolol maleate. Key words: glaucoma, beta-adrenoblockers, timolol maleate, Timolol gel.
Glaucoma is a chronic disease that requires constant treatment. Conservative treatment of glaucoma involves the administration of local antihypertensive drugs for a long time to maintain a normal level of intraocular pressure (IOP). The safety and good tolerability of antihypertensive drugs are of primary importance for patients’ compliance with doctor’s recommendations and the regimen for using these drugs and, as a result, maintaining the visual functions of patients with glaucoma [6, 15, 25]. The choice of drugs for conservative treatment of a patient with glaucoma depends on its form and concomitant diseases. It is also necessary to take into account the peculiarities of the mechanism of action, pharmacodynamics and composition of the prescribed drugs. Ophthalmic drugs can cause not only local, but also systemic adverse reactions as a result of absorption into the bloodstream [32]. In recent years, much attention has been paid in the literature to the concept of adherence to treatment [17]. It is directly related to the dosage regimen and duration of treatment. The more often during the day the patient must instill the drug, the greater the likelihood that the dosage regimen will be violated [18]. Appeared in the late 1970s. Beta blockers in the form of ophthalmic dosage forms have become a major step forward in the treatment of glaucoma. In recent years, beta-adrenergic receptor blockers have been considered the recommended drugs of first choice [3, 4]. The main place among them is timolol maleate [7]. When treated with local beta-blockers, both local and systemic side effects may be observed. The most common systemic manifestations include reactions from the respiratory and cardiovascular systems (fluctuations in heart rate and blood pressure). The severity and frequency of systemic side effects of local beta blockers are determined by the characteristics of their pharmacokinetics. When applied topically, timolol maleate does not undergo primary metabolism in the liver, which leads to its significant concentration in the blood plasma and an increased risk of systemic adverse reactions [14, 35]. After instillation, about 80% of the eye drops enter the systemic circulation. Considering that a 0.5% solution of timolol maleate is used 2 times a day, it can be assumed that this amount, corresponding to 200 mcg of the active substance, will increase the risk of developing serious adverse reactions [25]. One of the ways to reduce the frequency and severity of side effects of beta blockers is to prescribe their long-acting forms, in particular the long-acting form of timolol maleate. This allows you to achieve and maintain the optimal effective concentration of the active substance for a long time. In Russia, a multicenter study of a long-acting dosage form of timolol maleate was conducted in 1998 in 7 ophthalmology centers with the participation of 111 patients (161 eyes) with POAG or ocular hypertension. Patients were prescribed a 0.5% solution of long-acting timolol maleate (Timoptic-depot) 1 time per day. In case after 4 and 8 weeks. monotherapy with Timoptic-depot, the level of true IOP remained above 21 mm Hg. Art., patients were prescribed additional therapy. The effectiveness of Timoptic-depot monotherapy was assessed based on data on the level of ophthalmotonus after 4 weeks. use of the drug in those patients in whom monotherapy was ineffective (50 eyes), and IOP measurement data obtained during examination after 8 weeks. treatment (111 eyes). The average IOP level at the end of treatment with Timoptic-depot was 18.5±5.5 mmHg. Art. On average, the level of ophthalmotonus decreased by 7.3±5.1 mmHg. Art. (p<0.01) (Fig. 1). Visual field examination showed that during therapy for 12 weeks. the frequency of registration of absolute and relative scotomas has decreased. The maximum number of relative scotomas detected during examination of one eye also decreased. While using Timoptik-depot for 12 weeks. no significant change in blood pressure levels was observed. There was a moderately pronounced change in heart rate. Over the entire study period, clinically significant sinus bradycardia was detected in 6 (5.41%) patients. The use of Timoptik-depot 1 time per day made it possible to control the level of ophthalmotonus and reduce the amplitude of its fluctuations during the day. The prolonged form of timolol maleate had a less pronounced effect on heart rate compared to its aqueous solution [11]. The effectiveness and safety of Timoptic Depot were also studied in comparison with the effectiveness and safety of 0.5% betaxolol solution and 0.5% timolol maleate solution [1, 2]. The study included healthy volunteers and patients with primary open-angle glaucoma (POAG). A total of 230 people (368 eyes) were examined. After 8 weeks treatment, Timoptic-depot demonstrated the greatest effectiveness in comparison with 0.5% timolol solution and 0.5% betaxolol solution. Another long-acting formulation, Niolol gel 0.1%, which contains timolol in combination with a carbomer to deliver the active substance, has been studied in several studies in comparison with a 0.5% aqueous solution of timolol maleate [20, 26, 28, 33]. Local concentrations of both drugs were comparable (Fig. 2). It was noted that after 8 weeks. use of drugs, the reduction in IOP in both groups was comparable [26, 28]. The value of the residual decrease in IOP after more than 24 hours from the instillation of Niolol gel was statistically significant. Thus, the duration of action of the new form of timolol exceeded that of a 0.5% aqueous solution by 5 times [20]. A 4-week comparative study was also conducted in 32 patients (64 eyes) with POAG of a fixed combination of prostaglandin analogues and an aqueous solution of 0.5% timolol maleate (latanoprost + timolol, travoprost + timolol, bimatoprost + timolol) with an unfixed combination of prostaglandin analogues: latanoprost , travoprost and bimatoprost and 0.1% timolol in gel form. In all groups, the decrease in IOP levels was more pronounced and statistically significant (p<0.001) when prescribing a non-fixed combination with a prolonged form of timolol maleate. The administration of 0.1% timolol in gel form also increased the number of patients with daily IOP fluctuations ≤ 2 mmHg. Art. [12]. Regarding the frequency and severity of systemic side effects, comparative studies of 0.5% timolol solution and 0.1% timolol hydrogel showed that blood pressure, heart rate, changes in the QT interval and PR were less susceptible to changes when prescribing a long-acting form of timolol [1 , 2, 12, 16, 20, 21, 26, 28]. Along with this, some researchers report comparable safety for the two dosage forms of timolol [22]. Due to the slow absorption of timolol from this dosage form, it is determined in the blood plasma in lower concentrations than the usual solution of timolol maleate [5]. A number of studies have shown that the hypotensive effect with a single dose of a 0.1% extended-release form is comparable to or superior to the effect with a double dose of a 0.5% concentration of a regular solution of timolol maleate or superior [12, 20]. At the same time, the gel form of timolol maleate has a less pronounced effect on heart rate compared to its aqueous solution [1, 2, 5, 12, 13, 16, 19–21, 23, 26, 28, 34] (Fig. 2) . Thus, with similar effectiveness, the use of a prolonged form of timolol maleate is associated with fewer systemic side effects. This also helps reduce the cost of treatment [26]. A new drug, timolol maleate, which recently appeared on the market in the form of an eye gel, Oftan® Timogel, contains excipients: carbomer, sorbitol and polyvinyl alcohol. They made it possible to reduce the concentration of the active component (timolol maleate) without compromising its effectiveness and to increase the duration of the local hypotensive effect. As a result, local and systemic tolerability of the drug improved. During the development of the drug and in the post-marketing period, several randomized controlled as well as open observational clinical studies were conducted with a total participation of about 1200 patients [24, 26, 30]. According to the results obtained, Oftan® Timogel, when instilled 1 time per day, was not inferior in effectiveness to eye drops of 0.5% timolol maleate in a standard dosage 2 times per day (Fig. 4). It was also noted that a decrease in the concentration of the active component in the drug leads to a 5-10-fold decrease in the concentration of timolol in the blood plasma compared to the standard regimen of using 0.5% timolol eye drops or combined latanoprost and timolol eye drops [24, 29, 30 ]. A decrease in timolol plasma concentrations was directly correlated with a lower incidence of systemic side effects. In particular, it was found that Oftan® Timogel has a significantly less effect on heart rate than 0.5% timolol eye drops. The dynamics of heart rate during an orthostatic test was assessed [29, 30]. Normally, the heart rate increases after moving from a horizontal to a vertical position, and decreases after moving from a vertical to a horizontal position. This dependence persisted during an orthostatic test against the background of the use of Oftan® Timogel eye gel, while against the background of instillation of 0.5% timolol eye drops, a statistically significant depression of heart rate was observed compared to the initial data (Fig. 5). As is known, in most cases, benzalkonium chloride is used as a preservative for antihypertensive eye drops in various concentrations. The toxic effect of benzalkonium chloride depends on its concentration and exposure time and is most pronounced during long-term treatment, which is the treatment of glaucoma. Benzalkonium chloride in the composition of eye drops, with long-term use, has a damaging effect on the lipid layer of the tear film, reduces tear production, and also disrupts the structure of the corneal epithelium, promotes apoptosis of conjunctival goblet cells and increases the risk of chronic inflammatory diseases of the eye. Considering the above, patients with glaucoma are recommended to prescribe medications with the minimum possible concentration of a preservative or without it. The amount of preservative in Oftan® Timogel is 0.05 mg per 1 g of the drug, that is, reduced by half compared to a 0.5% solution of timolol maleate. This results in better local tolerance of the gel form of timolol compared to eye drops. When patients switched from 0.5% timolol eye drops to Oftan® Timogel, complaints of itching, lacrimation, burning and the feeling of a foreign body in the eye were significantly reduced [26]. When patients who had previously received treatment with Oftan® Timogel were prescribed eye drops of 0.05% timolol maleate, a greater severity of local adverse reactions was noted (Fig. 6). Prescribing beta-blockers as first-line drugs is justified from an economic and therapeutic point of view. However, the risk of systemic side effects should be taken into account, which may be overlooked by the doctor when prescribing topical drugs. Particular attention is required when treating elderly patients due to the presence of concomitant chronic diseases of the cardiovascular and respiratory systems. The use of long-acting forms of timolol maleate in patients with POAG and ocular hypertension leads to a pronounced decrease in IOP levels, which is comparable or even superior to that during therapy with a 0.5% aqueous solution of timolol maleate, as well as to less significant fluctuations in IOP during the day. Due to the low concentration in blood plasma, it is possible to reduce both the frequency and intensity of adverse reactions from the respiratory system and the vascular system. Single use during the day of prolonged dosage forms of timolol, such as Oftan® Timogel, helps to increase patient adherence to treatment, and therefore its effectiveness. Also noteworthy is the reduction in treatment costs due to the modified dosage regimen and reduced drug consumption. All of the above makes it possible to classify the long-acting dosage form of timolol maleate Oftan® Timogel as an effective and safe treatment for glaucoma, which has significant advantages over the existing forms of timolol maleate 0.25 and 0.5%.
Literature 1. Egorov E.A., Khlobystov A.A., Stavitskaya T.V., Brodskaya M.V., Bishele N.A. Comparative study of the effectiveness and safety of betaxolol, timolol maleate and timoptic-depot: Materials of the 11th scientific and practical. conf. “New technologies in eye microsurgery”, September 14–16, 2000, Orenburg. pp. 74–77. 2. Egorov E.A., Stavitskaya T.V., Khlobystov A.A. A comparative study of the effect of betaxolol, timolol maleate and timoptic depot on ophthalmotonus, ocular hemodynamics and myocardial conductivity: Proceedings of the VII Congress of Russian Ophthalmologists, May 16–20, 2000, Moscow. Part 1. P. 122. 3. Egorov E. A. et al. Ophthalmopharmacology. M.: GEOTAR-Med, 2004. 4. Erichev V.P. Main directions of antihypertensive treatment of patients with primary glaucoma // Rus. ophthalmol. magazine 2000. T. 1. No. 1. P. 18–21. 5. Erichev V.P., Yakubova L.V. Efficacy and safety of using timoptic depot in antihypertensive therapy of glaucoma // Bulletin of Ophthalmology. 1998. T. 114. No. 1. P. 8–9. 6. Kuroyedov A.V. Prospects for the use of combined antiglaucoma drugs (literature review) // Clinical ophthalmology. 2007. T. 8. No. 4. pp. 176–180. 7. Nesterov A.P. Current problems in glaucoma therapy: Proceedings of the symposium “The use of Fotil and Fotil Forte in the light of modern principles of glaucoma treatment.” M., 1996. pp. 3–4. 8. Nesterov A.P. Glaucoma. M.: Medicine, 1995. 9. Nesterov A.P. General assessment and choice of treatment methods for glaucoma;: Sat. scientific tr. "Physiology and pathology of intraocular pressure." M., 1987. pp. 60–68. 10. Nesterov A.P. Primary glaucoma. M.: Medicine, 1973. 11. Nesterov A.P., Egorov E.A., Astakhov Yu.S., Volkov V.V. and others. Timoptic-Depot: a multicenter study of effectiveness and safety // Medical Market. 1999. Vol. 2. No. 32. P. 39–41. 12. Nucci C., Varesi C., Martucci A., Cesareo M., Cedrone C., Mancino R., Cerulli L. Efficacy of timolol 0.1% gel and a prostaglandin analog in an unfixed combination compared to the corresponding fixed combinations / / Eur J Ophthalmol. 2013. Vol. 5. R. 683–689. 13. Dickstein K., Aarsland T. Comparison of the effects of aqueous and gellan ophthalmic timolol peak exercise performance in middle-aged men // Am-J-Ophthalmol. 1996 Apr. Vol. 121(4). R. 367–371. 14. Edeki TI, He H., Wood AJ Pharmacogenetic explanation for excessive beta-blockade following timolol eye drops. Potential for oralophthalmic drug interaction // J. Am. Med assoc. 1995. Vol. 274. R. 1611–1613. 15. European Glaucoma Society – Terminology & Guidelines for Glaucoma (European Guidelines). Glaucoma Society. 2nd ed. Savona Italy: Editrice DOGMA, 2003. Vol. 3. R. 3–26. 16. Uusitalo H., Ka..ho..nen M., Ropo A., Ma..enpa..a.. J., Bja..rnhall G., Hedenstro..m H., Turjanmaa V. Improved systemic safety and risk–benefit ratio of topical 0.1% timolol hydrogel compared with 0.5% timolol aqueous solution in the treatment of glaucoma // Graefe's Arch Clin Exp Ophthalmol. 17. Hitchings R. Impotance of Compliance in the management of glaucoma // Abstract book. Symposium “Glaucoma”, Rome, Italy, 1999. January 29-31. P. 20. 18. Hosoda M., Yamabayashi S., Furuta M., Tsukahara S. Do glaucoma patients use eye drops correctly? // J. of Glaucoma, June 1995. Vol. 4.No. 3. P. 202–206. 19. Kanellopoulos AJ, Perry HD, Donnenfeld ED Comparison of topical timolol gel to oral acetazolamide in the prophylaxis of viscoelastic-induced ocular // Cornea. 1997 Jan. Vol.16 (1). R. 12–15. 20. Mayer H., von der Ohe N. Efficacy of a novel hydrogel formulation in human volunteers // Ophthalmologica. 1996. Vol. 210(2). R. 101–103. 21. Nino J. et al. Cardiovascular effects of ophthalmic 0.5% timolol aqueous solution and 0.1% timolol hydrogel // Clin Physiol Funct Imaging. 2002 Jul. Vol. 22(4). R. 271–278. 22. Quaranta L. et al. Circadian intraocular pressure and blood pressure reduction with timolol 0.5% solution and timogel 0.1% in patients with primary open-angle glaucoma // J Clin Pharmacol. 2012 Oct. Vol. 52 (10). R. 1552–1557. 23. Rosenlund EF The intraocular pressure lowering effect of timolol in gel-forming solution // Acta-Ophthalmol-Scand. 1996 Apr. Vol. 74(2). R. 160–162. 24. Rouland J.-F. et al. Timolol 0.1% gel once daily versus conventional timolol 0.5% solution twice daily: A comparison of efficacy and safety // Ophthalmologica. 2002. Vol. 216. R. 449–454. 25. Schwartz GF Compliance and persistency in glaucoma follow-up treatment // Curr.Opin Ophthalmol. 2005. Vol. 16. R. 114–121. 26. Stankiewicz A. et al. A multicenter, observative, non-invasive study of the tolerance of Nyolol gel 0.1% in ocular hypertensive patients // Klinika Oczna. 2004. Vol. 106. R. 644–647. 27. Stewart WC, Sine C., Cate E., Minno GE, Hunt HH Daily cost of beta-adrenergic blocker therapy // Arch Ophthalmol. 1997, Jul. Vol. 115 (7). R. 853–856. 28. Uusitalo H. et al. Evaluation of efficacy and systemic side effects of topical 0.1% timolol gel and 0.5% aqueous timolol maleate // SOE Abstract. 1999. P 353. 29. Uusitalo H. et al. Efficacy and systemic side-effects of topical 0.5% timolol aqueous solution and 0.1% timolol hydrogel // Acta Ophthalmol Scand. 2005. Vol. 83. R. 723–728. 30. Uusitalo H. et al. Improved systemic safety and risk-benefit ratio of topical 0.1% timolol hydrogel compared with 0.5% timolol aqueous solution in the treatment of glaucoma // Graefe's Arch Clin Exp Ophthalmol. 2006. Vol. 244. R. 1491–1496. 31. Uusitalo R. Comparative study on the effect of two timolol preparations in glaucoma // VOX Glaucoma.1995. Vol. 17. No.1. P. 33–35. 32. Valuck JR, Perlman JI, Anderson C., Wortman GI Co-prescribing of medications used to treat obstructive lung disease, congestive heart failure and depression among users of topical beta blockers: estimates from three US Veterans Affairs Medical Centers // Pharmacoepidemiology and drug safety. 2001. Vol. 10. R. 511–516. 33. Von der Ohe N., Stark M., Mayer H., Brewitt H. How can the bioavailability of timolol be enhanced? A pharmacokinetic pilot study of novel hydrogels // Graefes Arch Clin Exp Ophthalmol. 1996 Jul. Vol. 234(7). R. 452–456. 34. Yamamoto T., Kitazawa Y., Azuma I., Tsukahara S., Nakashima M. Clinical evaluation of a new formula of timolol maleate (WP-934 ophthalmic solution) // Jpn-J-Ophthalmol. 1997 Jul-Aug. Vol. 41(4). R. 244–250. 35. Yarangumeli A., Kural G. // Expert Opin. Pharmacother. 2004 May. Vol. 5 (5). R. 1071–1081.
Glaucoma Treatment
Glaucoma is a specific optical neuropathy characterized by increased intraocular pressure (IOP) due to impaired production or outflow of aqueous humor (intraocular fluid) from the eye, changes in visual fields, disc excavation and atrophy of the optic nerve, decreased light sensitivity of the retina and the development of progressive optical neuropathy.
The physiological average IOP level is 15±1 mmHg. column and is regulated mainly due to the resistance of the trabecular network to the outflow of aqueous humor from the anterior chamber of the eye into Schlemm's canal.
The physiological level of IOP is maintained due to the equality of two components: the volume of fluid inflow formed by the ciliary processes and the volume of moisture outflow into Schlemm’s canal (average value 2.0-2.5 mm3 / min).
When any of these components change (the rate of moisture production or the rate of outflow), a change in the IOP gradient inevitably occurs, which underlies the pathophysiology of glaucoma.
Primary open-angle glaucoma - pathogenesis
The causes of trabeculopathy and subsequent blockade of the scleral sinus are not completely clear, however, it is known that involutional changes in the connective tissue structures of the sclera and ciliary muscle play an important role in the pathogenesis of open-angle glaucoma.
They are manifested by a violation of the ratio of collagen and elastin components, leading to increased density and decreased elasticity of connective tissue, mechanical displacement of the ciliary muscle towards the anterior chamber, and as a result, disruption of outflow along the uveoscleral pathway.
Thickening of trabecular plates, destruction of muscle fibers, narrowing of intertrabecular gaps generally reduces the permeability of the trabecular apparatus and, accordingly, leads to a persistent disruption of the physiological balance between the influx of fluid into the anterior chamber and the volume of its outflow through Schlemm’s canal. As a result, IOP can increase to 100 percent or more of the normal physiological level.
Constant disturbances in hydrodynamics, an increase in the pressure difference in the anterior chamber and Schlemm's canal leads to blockade of the scleral sinus and its narrowing, which in turn leads to compression of the trabecular apparatus and chronicity of existing pathological changes in the trabeculae, as well as compression of the optic nerve fibers at the site of their exit from the eyeball.
Chronic hemodynamic disturbances reduce retinal perfusion and increase the sensitivity of the optic nerve to fluctuations in ophthalmotonus.
Constant ischemia of the head and fibers of the optic nerve, together with compression of the retinal artery in the area of the optic disc, leads to progressive optic neuropathy as a result of the death of neurons and atrophy of retinal ganglion cells.
Primary angle-closure glaucoma - pathogenesis
Primary angle-closure glaucoma is characterized by partial or complete closure of the angle of the anterior chamber of the eye by the root of the iris.
Blockage of the anterior chamber angle occurs as a result of its closure by the thickened basal fold of the iris when the pupil dilates, which leads to even greater protrusion of the iris root, until the gradual complete closure of the anterior chamber angle and fusion of the iris root with its anterior wall.
Certain anatomical features predispose to the development of this form of glaucoma - a small anterior chamber with a narrow angle, a large lens, a thickened iris root.
Due to a decrease or complete closure of the anterior chamber angle, a mechanical obstacle arises in the path of aqueous humor outflow and the drainage function is disrupted - the root of the iris is “pressed” against the drainage network, increasingly closing the iridocorneal angle with the subsequent formation of adhesions.
Due to adhesions, the access of moisture to the drainage network is even more difficult, as a result, IOP increases in the affected eye. A change in the IOP gradient further triggers the same pathological cascade of reactions as in open-angle glaucoma, with compression and ischemia of the fibers of the optic nerve and retinal artery, which, in conditions of increased sensitivity of the nerve to fluctuations in ophthalmotonus, leads to pronounced excavation of the disc, atrophy of the ganglia and the development of progressive optic neuropathy .
Glaucoma - antihypertensive therapy
The primary goal of glaucoma treatment is to stabilize IOP to reduce the risk of optic nerve damage and the development of optic neuropathy. To achieve “goal pressure”, two groups of drugs are currently used:
Group 1 – drugs that reduce the production of aqueous humor
- selective and non-selective beta blockers - inhibit the production of aqueous humor by neutralizing the effect of catecholamines in beta receptors, thus helping to reduce IOP;
- carbonic anhydrase inhibitors - reduce the production of aqueous humor, slightly improve blood supply to the retina and optic nerve;
- agonists of central alpha-2 adrenergic receptors - improve the functions of the drainage system and reduce the production of aqueous humor.
Group 2 – drugs that increase the outflow of aqueous humor
- prostaglandins and analogues of prostaglandin F2-alpha - increase the outflow of aqueous humor both along the main and uveoscleral pathways due to interaction with specific receptors of the eyeball, destruction of collagen fibrils of the extracellular matrix, reduction in the thickness of the ciliary muscle and resistance to the outflow of aqueous humor.
Group 3 – fixed combinations with combined effects
- combinations of prostaglandins and beta blockers, used when monotherapy with antihypertensive drugs is ineffective, providing both a decrease in the production of aqueous humor due to the effect on beta receptors, and an increase in its outflow along the uveoscleral pathway due to the described mechanisms of interaction with FP receptors of the eyeball.
Glaucomatous optic neuropathy
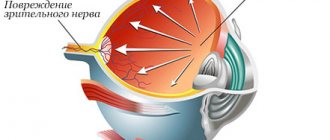
Glaucomatous optic neuropathy occurs in response to decreased tolerance of the optic nerve to increased IOP, characterized by progressive damage and apoptosis of optic nerve fibers as a result of chronic ischemia and a complex of metabolic disorders.
Although the exact mechanisms of glaucomatous optic nerve fiber atrophy remain unclear, it is assumed that the process begins with nerve fibers located around the fovea, subject to constant ischemia due to both impaired hydrodynamics and constant compression of the main retinal artery in the area of the central fovea. As the disease progresses, ischemia intensifies and the hydrodynamic balance is persistently disturbed, the lamina cribrosa of the sclera shifts backward, which further compresses the nerve fibers, promoting a gradual increase in the excavation of the optic disc and a narrowing of the neural ring, as well as the spread of the process to the peripheral parts. Concomitant circulatory disorders, microvascular disorders, changes in rheology and decreased retinal perfusion accelerate the process of apoptosis of the retinal ganglia and optic nerve head, ultimately leading to complete atrophy and blindness of the patient.
Glaucoma – neuroprotection
In order to prevent the progression of optic neuropathy, the second main goal of glaucoma therapy is neuroprotection, defined as interruption of the earliest processes underlying the ischemic cascade of reactions and apoptosis of nerve fibers.
The three main ways of implementing neuroprotection are inhibition of the damaging factor, increasing the resistance of the optic nerve to fluctuations in ophthalmotonus, and preventing atrophy of the optic nerve and retina. Neuroprotective therapy for glaucoma is aimed primarily at correcting metabolic disorders that occur in the head of the optic nerve during glaucoma.
In addition, the goal of treatment is to improve local microcirculation and tissue trophism, normalize the rheological properties of blood, and increase basal and collateral circulation.
However, neuroprotective therapy is effective only if the “target pressure” is achieved with the help of drug treatment, laser or surgical treatments (Russian National Guidelines on Glaucoma (2011). Therefore, against the background of antihypertensive therapy, the prescription of neuroprotectors is recommended for all patients.
Neuroprotectors are drugs that protect retinal neurons and optic nerve fibers by blocking apoptosis mechanisms in various ways, increase the resistance of nerve cells and neutralize various factors that increase the risk of their damage. Neuroprotectors are divided into groups of primary and secondary action.
Group 1 – Primary-acting drugs aimed at interrupting the processes of the ischemic cascade at an early stage
- GABA derivatives – increase resistance to hypoxia of nervous tissue due to nootropic and vasodilating effects;
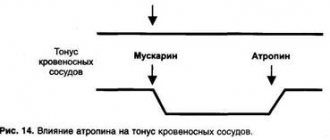
- voltage-gated calcium channel antagonists - act at the level of the neural synapse, blocking calcium channels of the presynaptic membrane, preventing excessive release of the neurotransmitter glutamate into the synaptic cleft and thus binding glutamate-chemoregulated ion channels of the postsynaptic membrane, preventing the entry of Ca++ ions into axons and providing direct neuroprotection .
Group 2 – Secondary-acting drugs aimed at interrupting delayed mechanisms of neuronal death
- antioxidants - causing the destruction of reactive oxygen species, inhibit the development of degradation processes in the trabecular apparatus and in the fibers of the optic nerve, and also neutralize free radicals;
- NO synthase inhibitors – block the release of excess NO synthase, which is released in excess by astrocytes, leading to apoptosis of retinal ganglion cells;
- neurotrophic factors and regulators of receptor structures - regulate the activity of nervous tissue due to the release of specific endogenous peptides;
- prostaglandin F2-alpha analogues and their fixed combinations with beta-blockers - the mechanism of neuroprotective action is believed to be due to the inhibition of xanthine oxidase, suppression of cyclooxygenase (COX-2) activity, stimulation of the production of endogenous prostaglandins PGE2, which protect neurons from damage and activation of F2alpha receptors (FP ) through the production of protein kinase.
Source: https://medstrana.com/articles/5538/
Open-angle glaucoma: modern approaches to drug therapy
Today, the collective term “glaucoma” unites a large group of diseases characterized by a constant or periodic increase in intraocular pressure, followed by the development of typical visual defects and optic nerve atrophy. These diseases, different in etiology, have a number of common features in pathogenesis, clinical picture and treatment methods.
A triad of signs characteristic of glaucoma:
- periodic or constant increase in the level of intraocular pressure (IOP) beyond the level tolerant for the optic nerve;
- development of glaucomatous optic neuropathy (GON) with subsequent atrophy (with excavation) of the optic nerve head (ONH);
- the occurrence of characteristic changes in the visual field (VF).
Glaucoma is one of the most severe forms of ophthalmopathology. Its socio-medical significance is also great: a high proportion among diseases of the organ of vision (in Russia: myopia - 19.1%; cataracts - 16.4%; glaucoma - 7%1); a high probability of developing blindness (in the structure of blindness and low vision, its share is 29.0%,2 second only to cataracts) and, as a consequence, disability of patients; high costs of public funds for their medical, social and domestic rehabilitation. And of course, a significant increase in morbidity.
According to the forecasts of the authoritative expert Quigley H., the number of patients with glaucoma in the world by 2010 was expected to be 66.5 million people, by 2020 - to increase to 79.6 million,2 and by 2030, according to calculations by Goldberg J ., to reach 120 million people.3 But already in 2003, at the Basel (Switzerland) international congress, well known to ophthalmologists, “Treatment of Glaucoma: Modern Aspects and Various Opinions,” the reports of specialists voiced completely different figures: 105 million patients with glaucoma in the world, blind for both eyes – 9.1 million people.
According to Professor E.S. Libman, in our country in 1965–1995. the frequency of blindness from glaucoma remained steadily at the level of 14–15% of the total number of blind people.4 Over 10 years (1998–2008) in the nosological structure of blindness and low vision, the share of glaucoma in Russia more than doubled - from 14 to 29%,5 becoming the undisputed leader.
Russian medical statistics on ophthalmological diseases continue to eloquently state the steady and stable increase in the prevalence of glaucoma (and not only due to the improvement of our diagnostics and monitoring). According to the country's chief ophthalmologist V.V. Neroev, the number of patients with glaucoma at the end of 2011 in 82 regions of the Russian Federation was 955,234 people (44,658 people more than in 2010), the average prevalence of glaucoma in the country was 83 per 10 thousand adults.6 B In 2014, these indicators were 955.2 per 100 thousand population (1.1 million cases).1
It should be added that the overall incidence is directly related to age: glaucoma occurs in 0.1% of patients aged 40-49 years, 2.8% - 60-69 years, 14.3% - over 80 years old.7 And what Among the numerous forms of this ophthalmic pathology, the most important is primary open-angle glaucoma (POAG), which makes up about 70% of all glaucomatous eye lesions8 and is characterized, as is known, by progressive optic neuropathy with specific changes in the optic nerve head and visual field against the background of instability and increased levels of ophthalmotonus .
On issues of pathophysiology
More than 60 years ago, our famous colleague Academician M.I. Averbakh described the situation with glaucoma simply and clearly: “The problem of glaucoma is a complete chaos, which is difficult to understand.” And today these words are largely relevant. But the complex pathogenesis of this disease is not fully understood. Experts believe that its pathophysiology includes genetic and biomechanical factors, hemodynamic disorders, changes in intracranial pressure (pressure gradient), changes in the drainage system, biochemical disorders, etc.
A reasoned scheme for the pathogenesis of primary glaucoma was proposed back in the last century by Academician A.P. Nesterov , becoming one of the founders of a new direction in ophthalmology - hydrostatics and hydrodynamics of the eye. He named the main links in the pathogenesis of primary glaucoma: heredity; general changes (hemodynamic and metabolic disorders, neuroendocrine disorders); local dystrophic changes; disturbances in the hydrodynamics of the eye (increased resistance to the outflow of intraocular fluid and increased ophthalmotonus); secondary vascular and dystrophic changes in the tissues of the eye and the optic nerve. The combination of all these factors leads to retention of chamber moisture and an increase in IOP. The circulation of moisture is hampered, its stagnation occurs, which leads to a deterioration in the nutrition of the avascular structures of the eye. Against the background of increased IOP and emerging circulatory disorders in the system supplying the optic nerve, glaucomatous optic neuropathy (GON) develops. Apoptosis of retinal ganglion cells is promoted by various factors: compression of the optic nerve axons in the openings of the cribriform plate (displaced posteriorly due to IOP), impaired blood supply to the head of the optic nerve, the formation of excess free radicals due to ischemia, and increased lipid peroxidation (LPO). Ischemia in nervous tissue is associated with inhibition of protein synthesis and activation of anaerobic glycolysis, disruption of the K-Na pump and depolarization of cell membranes. The response is the release of glutamate, which activates neurons through NMDA receptors, and the entry of excess calcium ions into the cell. An excessively high concentration of Ca2+ in cells triggers the activation of complex cascades of nucleases, proteases and lipases that affect intracellular proteins and lipids. The result is the formation of active free radicals and excessive amounts of nitric oxide (NO), which can contribute to the development of GON. As well as reactive oxygen species, which have a cytotoxic effect on the retina, optic nerve, and lead to destructive changes in the drainage system. Thus, through metabolic processes, both mechanical and vascular factors realize their effects.9-13
Stages of pathogenesis:
Deterioration of the outflow of intraocular fluid from the eyeball → increased IOP → deterioration of blood supply in the structures of the eye → the appearance of a zone of ischemia and hypoxia in places of deteriorated blood supply, which is reflected in the optic nerve → further increase in IOP, ischemia, hypoxia lead to apoptosis of retinal ganglion cells and blindness.
General principles of drug therapy
Despite all this diversity of pathogenetic factors, an important and primary condition for the successful treatment of glaucoma, including POAG, is the normalization of ophthalmotonus and its long-term stabilization at the target pressure level, and against the background of normalized IOP, correction of metabolic and hemodynamic disorders that contribute to the development and progression of glaucoma is carried out. optical neuropathy.
Table 1. Classification scheme of IOP level in glaucoma14
| IOP level | IOP tonometric, Pt | IOP true, P0 |
| Normal (A) | ≤ 25 mmHg Art. | ≤ 21 mmHg Art. |
| Moderately elevated (B) | 26≤ Pt ≤32 mm Hg. Art. | from 22 ≤ P0≤ 28 mm Hg. Art. |
| High (C) | ≥ 33 mmHg Art. | ≥ 29 mmHg Art. |
Today, intraocular pressure (IOP) can be reduced with medication, laser and surgery. Most often, treatment begins with the local use of antihypertensive drugs.
In the Federal clinical guidelines “Primary open-angle glaucoma”14 experts propose the following algorithm for the doctor’s actions to normalize the patient’s IOP:
Patient with a confirmed diagnosis of POAG → monotherapy with an antihypertensive drug → when target IOP is achieved → clinical observation.
Or:Patient with a confirmed diagnosis of POAG → monotherapy with an antihypertensive drug → target IOP not achieved or poor drug tolerability → change of drug or laser treatment → target IOP not achieved → addition of a 2nd drug or prescription of a fixed combination → target IOP not achieved or poor drug tolerability → other drug or laser treatment → target IOP not achieved or poor tolerability of drugs → surgical treatment, additional antihypertensive therapy.
Table 2. Main pharmacological groups of antihypertensive drugs and their mechanism of action
| Pharmacological group | Mechanism of action |
| Prostaglandins | improving the outflow of intraocular fluid |
| M-cholinomimetics | improving the outflow of intraocular fluid |
| Non-selective β-blockers | reducing the production of intraocular fluid |
| Selective β-blockers | reducing the production of intraocular fluid |
| α- and β-blockers | reducing the production of intraocular fluid |
| Carbonic anhydrase inhibitors | reducing the production of intraocular fluid |
| reducing the production of intraocular fluid | |
| reducing the production of intraocular fluid | |
| α2-selective adrenergic agonist | improving outflow and reducing the production of intraocular fluid |
When choosing a drug, it is important to follow the general principles of antihypertensive therapy: even before the start of treatment, the target pressure is determined (taking into account all the risk factors present in a particular patient and the initial IOP values); the effect of the prescribed antihypertensive regimen on each eye of the patient is assessed separately; treatment begins with monotherapy with the first-choice drug (if it is ineffective or poorly tolerated, replace it with drugs from another pharmacological group or with a combination drug); the adequacy of the achieved hypotensive effect is regularly checked by examining the state of the optic disc and visual functions. When carrying out combination therapy, you should not use more than two drugs (especially one pharmacological group) at the same time; It is preferable to use drugs in the form of fixed combinations.
When assessing drug exposure, it is necessary to take into account:
- type of influence on the hydrodynamics of the eye;
- degree of possible decrease in IOP level;
- presence of contraindications for use;
- portability;
- required frequency of use.
And remembering that this treatment is long-term, throughout the patient’s life, the doctor should not forget about tachyphylaxis and, to prevent its development, carry out a planned replacement of drugs.
The use of local antihypertensive drugs is possible not only in combination with each other, but also in combination with laser and surgical treatment methods.
Then, against the background of normalized IOP, correction of metabolic and hemodynamic disorders that contribute to the development and progression of glaucomatous optic neuropathy is prescribed.
Neuroprotective therapy
Modern knowledge about the etiopathogenetic mechanisms of glaucoma development has confirmed experts in the opinion that this multifactorial disease is neurodegenerative in nature and has much in common, for example, with Alzheimer's and Parkinson's diseases.
Glaucomatous optic neuropathy (GON) is a term that perhaps reflects the modern stage in understanding the processes of damage to the visual analyzer in glaucoma. GON is formed under the influence of many factors leading to apoptosis of retinal ganglion cells. There is also a mechanical disturbance of the axonal flow caused by increased IOP; and disruption of the blood supply to the ONH; and excess free radicals associated with ischemia and increased lipid peroxidation.15-16
As studies have shown, progressive optic neuropathy involves pathological changes in the visual fields and death of retinal ganglion cells, therefore, along with the antihypertensive component, a neuroprotective component is necessary in the treatment of a patient with this ophthalmopathology.17
Modern ophthalmology has a variety of drugs with neuroprotective effects. These are also direct-acting neuroprotectors. They directly protect retinal neurons and optic nerve fibers, blocking direct factors of cellular damage (increased concentrations of lipid peroxidation products and free radicals, Ca++ ions). And indirect-acting neuroprotectors, which indirectly have a protective effect and increase the resistance of various functional systems to decreased perfusion pressure in the vessels of the eye and hypoxia (drugs that improve microcirculation, rheological properties of blood, lower cholesterol levels in the blood, nootropic drugs).
The main means of neuroprotective therapy include NMDA receptor antagonists, antioxidants, peptide drugs, calcium channel blockers, alpha-2 agonists, carbonic anhydrase inhibitors, prostaglandin analogues, etc.
Federal recommendations10 draw the attention of doctors to the nicotinic ester of GABA - the drug Picamilon. This is the first domestic original drug with nootropic activity, synthesized back in 1969 at the All-Union Vitamin Research Institute and studied at the Research Institute of Pharmacology of the Russian Academy of Medical Sciences.
Due to its origin, Picamilon has the properties of both GABA (an inhibitory neurotransmitter of the central nervous system) and nicotinic acid (which has a vasodilator effect). It is quickly absorbed from the gastrointestinal tract; unlike GABA, it easily penetrates the blood-brain barrier (BBB). It has a nootropic (improving metabolism and brain function) and short-term vasodilating effect.19
Over the years of its use (introduced in 1986), a significant evidence base of its effectiveness has been accumulated. Picamilon has proven itself as a means of metabolic therapy, combining pronounced vasoactive properties, nootropic effects and a tranquilizing effect.
Easily penetrating the BBB, Picamilon influences the mechanisms of neurovasomotor regulation, stimulating cerebral circulation (due to a decrease in cerebral vascular tone in the system of carotid and vertebral arteries, pial arterioles, an increase in the volumetric velocity of cerebral blood flow, a pronounced central depressive effect on the reflex contractile reactions of cerebral vessels and somatosympathetic reflexes). As GABA accumulates in brain tissue, the mechanisms of synaptic transmission are gradually optimized and neurodynamic processes in the central nervous system are normalized. Thanks to this, positive adaptive changes are formed in the most complex morphofunctional system of the visual analyzer.20
Picamilon has the following properties:
- improves brain productivity;
- increases endurance in relation to mental stress;
- has an antioxidant effect;
- improves blood microcirculation inside the eyes.
Here is one of the many studies of Picamilon. He was held at the State Budgetary Healthcare Institution of the Moscow Region Monika named after. M.F. Vladimirsky (Professor A.A. Ryabtseva), where 116 patients (aged from 56 to 80 years) were under observation.20 All patients (with similar disease indicators and complaints) received local antihypertensive therapy instillations in both eyes (timolol 0, 5% 2 times a day and brinzolamide 1 time a day). But some patients (group 2) received Picamilon at a dose of 50 mg 2 times a day for 1.5 months. The effectiveness of the treatment was assessed by the dynamics of visual acuity, perimetry, indicators of electrophysiological studies and the picture of the fundus.
This experience of using Picamilon as a means of metabolic therapy showed its positive effect on the hydrodynamic parameters of the eye in patients with POAG: a decrease in true IOP, an increase in the coefficient of ease of outflow, an increase in the secretion of intraocular fluid, a decrease in the Becker coefficient. The researchers noted a significant improvement in visual acuity (VA) from 0.37 ± 0.04 to 0.44 ± 0.05 in patients in the Picamilon group. And also after a course of taking Picamilon, a tendency towards an increase in light sensitivity (or a decrease in dark adaptation time) was revealed, which indicated an increase in the functional activity of the retina and optic nerve. The recommended duration of a course of taking Picamilon is from 1.5 to 3 months.
Thus, one of the most important problems of ophthalmology - the treatment of patients with primary open-angle glaucoma - requires a comprehensive approach from the doctor, including not only therapy aimed at normalizing intraocular pressure, but also neuroprotective therapy, which allows preserving axons that have not yet died, but are already experiencing the harmful effects of ischemia ganglion nerve cells. To correct disorders of the functional state of the visual analyzer, the domestic drug Picamilon, tested in many years of clinical practice by Russian doctors and available (including at a price) to their patients, can be successfully used.
Bibliography
1Neroev V.V. Organization of ophthalmological care for the population of the Russian Federation. Bulletin of Ophthalmology. 130 (6), 2014, p. 8–12.
2Quigley HA, Broman AT The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006, vol. 90, p. 262–267.
3Goldberg I. Glaucoma in the 21st Century. I. Goldberg // Hartcourt. Health Communication / London: Mosby Int., 2000, p. 4–8.
4Libman E.S. Report at the Congress of Russian Ophthalmologists, 2005/E.S. Libman // Newspaper “Oculist”. No. 4, 2005, p. 1.
5Libman E.S. Epidemiological characteristics of glaucoma / E.S. Libman // Glaucoma. No. 1, 2009, p. 2–3.
6Neroev V.V., Avdeev R.V., Kiseleva O.A., Bessmertny A.M. Selected results of an epidemiological study on glaucoma for 2011. Ophthalmological statements. T. 7, No. 2, 2014, p. 4–8.
7Tham YC, Li X., Wong TY, Quigley HA, Aung T., Cheng CY Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014, 121, p. 2081–2090.
8Alikova T.T., Alikova Z.R., Fidarova K.K., Yakhyaeva Z.I. Glaucoma incidence rate and follow-up of patients: regional features. International Journal of Applied and Basic Research. No. 8-2, 2020, p. 149–152.
9Nesterov A.P. Pathogenesis and problems of pathogenetic treatment of glaucoma // Clinical ophthalmology. No. 4 (2), 2003, p. 47–49.
10Nesterov A.P. Glaucoma. – M. LLC “Medical Information Agency”, 2008. 360 p.
11Marjanovic I., Milic N., Martines A. The impact of intraocular pressure reduction on retrobulbar hemodinamic parameters in patients with open-angle glaucoma // Eur. J. Ophthalmol. 2012, no. 225 (1), p. 77–82.
12Osborne NN, Wood JP, Chidlow G. et al. Ganglion cell death in glaucoma: what do we really know? // Br. J. Ophthalmol. 1999, vol. 83, no. 8, p. 980–986.
13Erichev V.P. Antihypertensive therapy of primary open-angle glaucoma with fixed combinations of drugs (methodological recommendations). M. April, 2012, 21 p.
14Federal clinical guidelines. Primary open-angle glaucoma. 2020. https://bz.medvestnik.ru/nosology/Pervichnaya-otkrytougolnaya glaukoma.html/recomendations/classification.
15Osborne NN, Wood JP, Chidlow G. et al. Ganglion cell death in glaucoma: what do we really know? Br. J. Ophthalmol. 1999, vol. 83, no. 8, p. 980–986.
16Kurysheva N.I. Glaucoma optic neuropathy. M. MEDpress-inform, 2006. 136 p.
17Egorov E.A., Brezhnev A.Yu., Egorov A.E. Neuroprotection in glaucoma: modern possibilities and prospects. RMJ "Clinical Ophthalmology" No. 2 from 29.05, 2014, p. 108.
18Picamilon is a metabolic cerebrovascular agent and nootropic. Application in medical practice. Moscow. 2002, 48 p.
19Rational pharmacotherapy in ophthalmology. Guide for practicing physicians. Under general ed. E.A. Egorova. M., Litera. 2004, p. 435.
20Ryabtseva A.A. Experience of neurometabolic treatment of primary open-angle glaucoma: approaches to stabilization of visual functions. https://medvestnik.ru/content/medarticles/Opyt-neirometabolicheskogo-lecheniya-pervichnoi-otkrytougolnoi-glaukomy-podhody-k-stabilizacii-zritelnyh-funkcii.html.