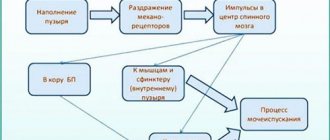
Fibrosis of the prostate gland, otherwise prostate sclerosis.
A pathological process manifested as the proliferation of connective tissue and the development of scar changes.
During these changes, the prostate shrinks.
This leads to compression of the urethral canal and disruption of the deurination process.
The pathology can also occur under the name paraurethral fibrosis of the prostate.
Clinical picture of prostate fibrosis
Prostate fibrosis and pathological changes against its background provoke the following symptoms:
- Pain during ejaculation, discomfort during intercourse.
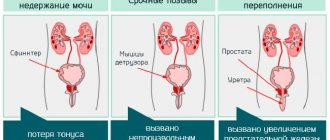
- Disruption of the urination process, which is accompanied by difficulty in deurination (the patient strains the pelvic muscles, trying to completely empty the bladder).
- Increased amount of urination (pollakiuria). Characterized by a significantly higher frequency of emptying the bladder with predominantly small amounts of urine.
- The development of nocturia is an increase in the amount of urination at night.
- Intermittent deurination (sluggish stream of urine, accompanied by bifurcation, changes direction).
- Bladder fullness with acute urinary retention. Stagnation of urine has a negative effect on the upper urinary tract, which leads to the development of postrenal renal failure.
- Pain syndrome localized in the groin and rectum. May get worse when going to the toilet.
Other signs of prostate fibrosis include the development of residual urine.
Residual urine should be considered the volume of urine that remains in the bladder after urination.
Symptoms of prostate stones
Single and small calcifications usually do not manifest themselves in any way. A man can carry them in the prostate all his life without experiencing discomfort. Multiple and infected stones cause problems. They manifest themselves as pain in the lower abdomen, penis, perineum, scrotum, sacrum . Stones cause chronic pelvic pain, which is constantly present and literally plagues a man. Discomfort increases during physical activity or prolonged sitting. In some cases, urination becomes difficult. Scarlet blood may appear in urine and semen.
Sometimes stones can be felt independently through the rectum. When pressing on the prostate, an unpleasant crunching sound (crepitus) is heard and pain is possible.
Types of prostate fibrosis
The clinical picture and prognosis of the disease will depend on the severity of the pathological process and its duration.
Thus, small focal fibrosis of the prostate can remain in an asymptomatic state for quite a long time.
Today in urological practice it is customary to distinguish the following types and forms of the disease:
- Focal fibrosis of the prostate. There is an increase in the concentration of cells, which leads to proliferation of the gland (in practice, focal fibrosis is often confused with chronic prostatitis).
- Fibrosis with parenchymal atrophy. Accompanied by a decrease in the concentration of cells and the volume of glandular tissue.
- Sclerosis of the prostate with simultaneous development of nodular hyperplasia. There is a proliferation of glandular tissue against the background of an increase in cells.
- Fibrosis with the development of cystic formations. The cyst is a closed capsule of epithelial tissue, may consist of one or more chambers and have elastic or viscous contents.
- Prostate cirrhosis. It is characterized by the replacement of normal prostate tissue with pathological tissue with subsequent disruption of its structure.
Other forms of fibrosis may be accompanied by an infectious process or an allergic reaction, and may not be a consequence of prostatitis.
Features of the structure of the prostate
The content of the article
The prostate gland (in Latin - prostata) is a special organ belonging to the male reproductive system. It is located on the pelvic floor, directly below the bladder, posterior to the symphysis pubis.
Prostate
The prostate consists of 2 lateral lobes connected by a node, which, as it grows, can form the so-called third lobe. The urethra and ejaculation tubes pass through the prostate parenchyma.
The prostate parenchyma consists of approximately 50-60 alveolar-urethral glands, forming clusters, separated from each other by a stroma consisting of smooth muscle and connective tissue. The clusters have their own drainage lines leading to the urethra in the area of the spermatic hill. According to the theory of teaching. McNeil, 4 zones can be distinguished inside the prostate, which differ in embryonic, morphological and functional origin:
- the central zone covering 25% of the prostate volume. The release lines pass through this area,
- transition zone, constituting 5-10% of the prostate volume,
- peripheral zone covering 70% of the organ volume,
- anterior commissure, consisting exclusively of fibromuscular stroma, completely devoid of glandular intertwining.
These areas are susceptible to various pathological processes.
- Prostate cancer most often develops from the peripheral zone (75% of cases), much less often from the transition zone (20%) and the central zone (5%).
- Prostate adenoma, on the contrary, forms in the transition zone (lateral lobes of the adenoma) or additionally in the central zone (middle and third lobes).
The prostate is an organ that is influenced by male sex hormones. Its growth and development depend on testosterone. The production of this hormone in the testicular Leydig cells is regulated by the hypothalamic-pituitary-testicular axis.
The hypothalamus produces luteinizing hormone releasing hormone (LH-RH), which stimulates the pituitary gland to produce luteinizing hormone (LH). On the other hand, LH, after binding to Leydig cell receptors, stimulates testosterone biosynthesis. There are two forms of testosterone in the blood: free and bound to globulin.
Free testosterone enters the glandular cells of the prostate, where, under the action of 5-alpha reductase, it is transformed into the active form of dihydrotestosterone (DHT). By binding to receptors in cell nuclei, DHT causes the synthesis of m-RNA and proteins, which leads to the growth and proliferation of prostate cells.
Causes of prostate fibrosis
What are the main causes of prostate fibrosis?
The main reason why prostate sclerosis may develop is long-term prostatitis (inflammation of the prostate gland).
More than 15% of men around the world have experienced prostate inflammation at least once in their lives.
The following phenomena can be provoking factors for fibrosis:
- Urethroprostatic reflux. It is often observed with increased intraurethral pressure, accompanied by the reflux of urine from the urethra into the efferent tubules of the prostate. As a result, an inflammatory process that is not associated with bacterial etiology develops.
- Various types of surgery previously performed on the prostate gland.
- Atherosclerotic lesion of the prostate gland, which is accompanied by the development of cholesterol plaques in the vessels of the prostate.
- Hormonal imbalance accompanied by testosterone deficiency.
- Acute inflammation of the prostate caused by bacterial pathogenic microflora.
Reviews about the treatment
Methods for crushing prostate stones are questionable, there are no clear positive reviews, as well as about dissolving them using conservative methods. The men undergo a course of treatment, the condition improves, but the formations remain in place. An example of a doctor's opinion is below.
Below is the answer of a doctor from the multidisciplinary “Preobrazhenskaya Clinic” in Yekaterinburg:
Reviews regarding crushing stones using shock wave therapy:
And some men categorically do not recommend UVT due to side effects: inflammation of the prostate worsens, pain increases, and premature ejaculation occurs.
Regarding crushing calcifications with a laser:
Diagnostic measures for prostate fibrosis
To make an accurate diagnosis and prescribe effective treatment, the following diagnostic procedures are used:
- Collecting anamnesis, analyzing the clinical picture and patient complaints. The doctor should ask the patient about his state of health, the severity of symptoms, and its nature. Additionally, the doctor asks you to fill out a special IPSS questionnaire, which will determine the quality of urination and identify disorders in deurination.
- General laboratory examination of urine. It is prescribed for the determination of leukocytes, protein, glucose, and infectious microorganisms in urine. The urine of a healthy person does not contain ketones, nitrites and blood components (for example, hemoglobin), as well as protein molecules. Also, a urine test can reveal the ongoing inflammatory process.
- General laboratory blood test. Conducted to calculate the concentration of erythrocytes, as well as their sedimentation rate. Based on the results of the analysis, one can judge the inflammatory reaction and its severity, as well as determine the state of the immune system.
- Biochemical blood test. Allows you to determine the functional state of internal organs and evaluate the functioning of the immune system. The results of the analysis make it possible to understand the processes occurring in the body, including metabolism.
- Microbiological culture of urine. Allows you to determine the bacteriological composition of urine and identify the pathogenic microorganism that provoked the inflammatory reaction in the urethral canal.
- Ultrasound examination of the bladder and kidneys. It is prescribed for the purpose of visualizing the condition of the kidneys, their size, and allows diagnosing stagnation of urine.
- TRUS of the prostate gland. TRUS of the prostate is not able to determine fibrosis with 100% accuracy, but will give a completely informative conclusion about the shape of the gland, its volume, deformations and structure.
Prostate fibrosis is often visualized on ultrasound as hyperechoic areas.
To fully diagnose fibrotic lesions of the prostate gland, it is necessary to conduct a complete urological examination.
How to exclude the development of an oncological process or detect it at the earliest stages?
A PSA blood test (prostate-specific antigen, a tumor marker) and a rectal ultrasound examination are prescribed.
If the development of an atypical process is suspected, a biopsy of the suspected tissue is performed.
Cystoscopy may also be prescribed.
Changes in the prostate gland that are not detected in a timely manner can provoke a number of complications.
Even benign changes disrupt the deurination process and contribute to the accumulation of urine in the bladder.
Urine accumulation significantly increases the risk of bladder stones, urinary tract infections, and a number of more serious complications.
Diagnosis of prostate hyperplasia
To assess the severity of LUTS in Europe, the IPSS (International Prostate Symptom Score) questionnaire is used, containing 7 questions about urinary symptoms.
The sum of points from the answers allows you to preliminary assess the severity of urination disorders:
- 0-7 points – minor symptoms;
- 8-19 points – symptoms of moderate severity;
- 20-35 points – severe symptoms.
The questionnaire is accompanied by a question about the quality of life at the current intensity of LUTS (QoL - quality of life).
In the clinical course of benign prostatic hyperplasia, the following IV stages can be distinguished:
- I – asymptomatic stage: prostate enlargement without clinical manifestations. Tubular flow is normal during this period.
- II – stage of irritation: prostate enlargement with variable symptoms of urinary tract irritation (periodic pollakiuria, urge). Tubular blood flow remains normal or slightly impaired. Ultrasound does not show urine residue in the bladder.
- III – stage of urinary retention: obvious enlargement of the prostate with constant symptoms of irritation and obstruction. The maximum flow in the tube is less than 10 ml/s, and the shape of the voiding curve is irregular. There is urine in the bladder.
- IV – stage of decompensation: a significant amount of urine in the bladder. Urinary retention and paradoxical wetting, that is, excessive flow of urine from a full bladder, may occur. There are complications from the bladder (clear trabecularities, pseudodiversions, urolithiasis), and the upper urinary tract is dilated (ureters and the calyceal-pelvic system). Renal failure and uremia develop.
To make a diagnosis, it is necessary to collect the patient's medical history, perform a physical examination, perform laboratory tests, transcleral ultrasound and uroflowmetry.
The history should indicate not only LUTS, but also concomitant and acquired diseases. An important addition to the interview is the completion of the IPSS questionnaire.
Physical examination includes palpation of the entire abdomen and digital rectal examination (DRE).
Signs of benign prostatic hyperplasia with PRE are:
- symmetrical enlargement of the prostate;
- homogeneous elastic consistency;
- smooth surface;
- as a rule, there is no pain on palpation.
Laboratory tests should first determine the concentration of PSA (prostate specific antigen) in the blood serum. It is a glycoprotein produced by the epithelial cells of the prostate gland. Functionally, PSA is a protease that liquefies sperm by digesting high molecular weight proteins included in the ejaculate.
Serum PSA (prostate specific antigen) concentration
PSA, released by glandular cells, enters the sperm first, with only a small amount entering the bloodstream.
Although an increase in serum PSA levels makes the urologist suspect prostate cancer, it should be remembered that an increase in the concentration of this antigen also occurs in acute and chronic prostatitis and large adenomas.
A transient rise in PSA may occur as a result of mechanical irritation of the prostate following bladder catheterization, cystoscopy, transurethral procedures, and prostate biopsy. Some medications can also change PSA levels. An increase in concentration occurs under the influence of drugs such as: Isoptin, Isoket, Diltiazem, while a decrease in level is observed during treatment with Proscar.
Most laboratories accept serum PSA standards as 0-4 ng/ml. However, taking into account observations that the PSA level is influenced by the age of the subject, age standards were introduced:
- 40-49 years – 2.5 ng/ml;
- 50-54 years – 3.7 ng/ml;
- 55-59 years – 4.0 ng/ml;
- 60-64 years – 5.4 ng/ml;
- 62-69 years old – 6.2 ng/ml;
- 70-74 years – 6.6 ng/ml.
In addition to the PSA, other laboratory tests are recommended to help assess general condition (blood count, glucose level), kidney function (urea, creatinine), and detect urinary tract infection (urinalysis and culture).
Transcapital ultrasound is useful in assessing post-void urinary retention, approximating the size of the prostate, and assessing the health of the kidneys and bladder.
Uroflowmetry allows you to assess the degree of bladder obstruction and detrusor capacity.
Uroflowmetry
If it is difficult to make a correct diagnosis, additional urography, cystoscopy, complete urodynamic examination, transrectal ultrasound (TRUS) and prostate biopsy should be performed.
How is prostate fibrosis treated?
Treatment of prostate fibrosis involves exclusively surgical methods.
Especially when it comes to late stages of pathology.
Unfortunately, medications or massage for prostate fibrosis are ineffective.
They cannot eliminate the active proliferation of connective tissue.
The use of drug treatment is possible only in the earliest stages of fibrosis development.
Various antibacterial drugs, NSAIDs, and painkillers may be prescribed.
Removal of prostate fibrosis is prescribed in the following cases:
- Retention of urination, which is accompanied by acute pain, a feeling of heaviness in the lower abdomen, and deterioration in general well-being.
- With fibrosis of the prostate, which is accompanied by the formation of stones in the bladder.
- When a protrusion of the urinary wall is detected.
- When there is an acute disturbance of the outflow of urine.
- In case of diagnosing congenital dilatation of the ureter.
- With the development of acute renal failure against the background of prostate sclerosis.
- Reflux of the ureter, which is accompanied by the reflux of urine from the urethral tract into the vesicula seminalis (seminal vesicles).
Diagnostics
Men usually go to the urologist when inflammation occurs and calculous prostatitis develops with all the corresponding symptoms. The doctor interviews the patient, palpates the prostate through the rectum, prescribes tests of urine, blood, prostate juice using the PCR method, uroflowmetry, and survey urography.
Calcifications are diagnosed using TRUS . Theoretically, they can also be detected through the abdomen with a sensor, but not all of them, so TRUS is preferable.
In photographs, calcifications appear as light dots or groups of them. These are areas of increased echogenicity - dense, therefore they reflect waves well. It is necessary to differentiate calcifications from foci of fibrosis. If in doubt, it is better to take an x-ray. The stones will be clearly visible on the x-ray. Examples of calcifications on ultrasound images are presented below.
Multiple calcifications
Single calcifications
Prostate without calcifications
Methods of surgical intervention for prostate fibrosis
Transurethral resection of the prostate
It is a urosurgical method for treating the prostate and bladder.
Often found under the abbreviated name TUR.
Transurethral resection (TUR) is a minimally invasive surgical procedure.
The manipulation is carried out using a resectoscope.
In medicine, a distinction is made between transurethral resection of the bladder (TUR-B or TURB) and transurethral resection of the prostate (TUR-P or TURP).
TUR-P is used to remove obstructions that block the flow of urine from the male prostate gland.
TUR-B is used to treat superficial bladder cancer.
In the TUR-P procedure, the surgeon removes prostate tissue that has become abnormal without making an external cut in the connective tissue.
Before undergoing transurethral resection of the prostate, the patient must temporarily stop taking certain medications to avoid complications.
These drugs include blood thinners such as acetylsalicylic acid and antidiabetic medications.
The drugs increase the risk of bleeding or metabolic acidosis.
In addition, a urinary tract infection must be ruled out before surgery.
If they are present, a course of antibiotics is first prescribed.
Treatment is carried out under epidural or spinal anesthesia.
At the beginning of the prostate resection procedure, the surgeon inserts a continuously irrigated resectoscope into the prostate through the urethral canal.
Pathologically changed tissue is removed using a high-frequency current loop.
Transurethral resection of the prostate gland can be performed in several ways - monopolar and bipolar.
The monopolar method uses a salt-free solution, while the bipolar method uses physiological saline as the irrigation solution.
The bipolar method is considered the safest, since it greatly reduces the risk of bleeding.
In most cases, resection of the prostate using the TUR method is successful, normalizes urination, and relieves all unpleasant symptoms associated with fibrosis of the gland.
As a rule, people try to resort to this method of surgery more often.
The surgical intervention is well tolerated by patients and is accompanied by a comfortable recovery period.
In rare cases, a number of complications associated with the operation may develop.
Further possible risks of transurethral resection include retrograde ejaculations, bladder neck sclerosis, testicular inflammation, and epididymitis.
Some patients also suffer from temporary erectile dysfunction.
Resection of the gland with direct surgical access
This method of surgical intervention is used only in cases of urgent need, when minimally invasive manipulations are inappropriate.
During the procedure, an incision is made in the soft tissue of the peritoneum, and if necessary, the bladder is opened.
This method of therapy is characterized by greater trauma.
The anesthesia used is spinal anesthesia or general anesthesia (as indicated).
This method of therapy has a fairly long recovery period for the patient.
There is also a wide range of possible complications: infections, bleeding, extensive hematomas.
For people with cardiovascular disease, open surgery under general anesthesia can lead to poor cardiovascular function.
There is also a risk of cardiac arrest.
Important! Treatment of prostate fibrosis with folk remedies is strictly prohibited. Such methods are not only ineffective, but can also worsen health.
When discussing morphological changes in prostate tissue during benign hyperplasia (BPH), it is first necessary to dwell on issues of terminology, since the abundance of synonyms for this disease creates significant difficulties in understanding the essence of the pathological process.
It is obvious to practicing urologists that the deterioration of urination in BPH (infravesical obstruction, decreased volumetric flow rate of urination, increased amount of residual urine) is due to an increase in the volume of the prostate gland. An increase in the volume of an organ, tissue, cell, intracellular organelles in general pathology is referred to as hypertrophy. This process is realized by an increase in the number of constituent components, called hyperplasia. In other words, an increase in the volume of an organ occurs either due to an increase in the volume of its constituent cellular elements (hypertrophy of cellular elements), or due to an increase in their number (hyperplasia of cellular elements). In turn, cellular elements can increase in volume due to an increase in the number or volume of intracellular organelles [1].
In fact, the basis of any type of hypertrophy is the process of hyperplasia, the only difference is what is taken as the starting point. The exception is cases when an increase in organ volume occurs due to tumor growth, benign or malignant. These cases are not included in the concept of hypertrophy. Consequently, the term prostatic hypertrophy has equal rights with the term prostatic hyperplasia, however, the latter term is preferable because it better reveals the essence of the pathological process in the prostate gland.
Currently, the vast majority of urologists and even pathologists unreasonably use the term prostate adenoma, thus classifying this disease as a group of tumor processes (since adenoma is a benign tumor developing from the glandular epithelium) [2]. At the same time, it is known that in the vast majority of cases, both benign and malignant tumors develop monocentrically. Polycentric tumor growth is extremely rare. With BPH, a urologist performing surgery almost always finds many nodes of varying sizes in the prostate gland. This indicates the polycentric development of the pathological process in this disease. Naturally, the question arises: what underlies the formation of these nodes, what do they represent?
The results of research conducted over many years at the Research Institute of Urology made it possible to determine the main stages of the morphogenesis of hyperplastic nodes developing in prostate tissue with BPH.
It is well known that the prostate gland consists of parenchyma, represented by glandular tissue, and stroma, which has a fibromuscular structure. It is characteristic that the cellular elements of the stroma have morphological characteristics of both a fibroblast cell of the connective tissue proper and a leiomyocyte of a smooth muscle cell [3]. Nodular structures, leading to an increase in the volume of the organ, in most cases have a glandular structure and go through a series of successive stages in their development. The transition from one stage to another is accompanied by the appearance of a new qualitative characteristic.
The process begins with the formation of a structure consisting of 2-3 glands tightly adjacent to each other; the proliferative center of the first stage of development is formed (Figure 1). In the adjacent parts of the organ, the gland retains its normal structure. The stromal elements surrounding the newly formed structure become denser, and van Gieson picrofuchsin staining shows that the myofibrillar stroma is transformed into coarse fibrous connective tissue, losing the signs of smooth muscle cells.
Further development of the process leads to a significant increase in the number of acinar structures (hyperplasia). In this way, microscopic nodules are formed, in which increased proliferation of glandular tissue occurs. This is evidence of the formation of stage II of the development of the proliferative center (Figure 2). The stromal elements become denser to an even greater extent, and therefore a false picture of the formation of a connective tissue capsule around the proliferative center is created. Serial sections, however, show that these proliferative centers have a well-defined radial structure similar to the Samba proliferative centers in the mammary gland, and the connective tissue fibers surrounding the proliferative center are intimately connected with the connective tissue framework inside it.
|
|
The progression of the pathological process is accompanied by the formation of additional foci of proliferation in the peripheral parts of the proliferative centers, i.e. unique daughter proliferative centers. This qualitative sign, the appearance of daughter foci of proliferation, is a morphological manifestation of the formation of the third stage of development of the proliferative center (Figure 3).
The constant progression of the proliferative activity of acinar structures greatly impedes the outflow of secretions from the prostate acini, which form the proliferative center, which leads to their cystic expansion. It is this qualitative S sign that indicates that the pathological process has entered the next, IV stage of development of proliferative centers (Figure 4).
|
|
In the future, the situation becomes even more aggravated: the entire proliferative center, or most of it, consists of such cystically dilated acini. The glandular cells lining them spread across the basement membrane, flatten and atrophy. Cystic expansion of acinar structures with atrophy of the epithelium lining them manifests the terminal, V stage of development of proliferative centers in BPH (Figures 5, 6).
|
|
Intra-acinar disruption of the outflow of secretions due to the proliferative process, already in the early stages of its development, can lead to the formation of stones inside the acini.
Thus, the sequence of morphogenetic stages of development of proliferative centers is as follows:
- Stage I: formation of a focus consisting of 2-3 acinar structures tightly adjacent to each other;
- Stage II: a sharp increase in the number of glandular structures forming the proliferative center;
- Stage III: the appearance of proliferative foci of proliferation in the peripheral parts;
- Stage IV is a violation of the outflow of secretions in single acinar structures that make up the proliferative center, with their cystic expansion;
- Stage V, all acini forming the proliferative center, or the vast majority of them, are cystically dilated, and the epithelium lining them is atrophied.
This path of development of the proliferative process in the glandular component of the prostate gland can be traced in most cases. However, some deviations are possible. Various variants of these deviations are called atypical hyperplasia. The term atypical hyperplasia does not at all imply that this process is related to oncological diseases, but only emphasizes the atypicality of the development of the hyperplastic process itself. According to our data, atypical forms of glandular prostate hyperplasia occur in 8.7% of all cases of glandular hyperplasia.
Among the atypical forms of glandular hyperplasia, basal cell hyperplasia occurs with the highest frequency. Its frequency is 86.3% of all atypical hyperplasias. With this form, in individual proliferative centers, foci are revealed in which glandular structures are formed by basal cells. Proliferations from basal cells can be in the form of solid cords, or they can appear as segmental proliferations within acinar structures.
Other variants of atypical hyperplasia are much less common. Thus, adenosis, the next most common form of atypical hyperplasia, accounts for 7.6% of cases of atypical hyperplasia. With this form of glandular hyperplasia, a proliferation of small glandular structures is observed in certain areas of the prostate gland. These glands are located chaotically and are separated from each other by thin layers of connective tissue. If the stroma in the foci of adenosis significantly dominates the parenchyma, then this variant is called fibrosing adenosis.
Another form of atypical hyperplasia is the so-called cribriform hyperplasia, when proliferating cells form a lattice-like structure. This form is quite rare (2.4%) and requires differential diagnosis with cribriform (cribrous) prostate cancer.
Phylloid hyperplasia has the lowest incidence (1.3%). It manifests itself in the formation of narrow slits in the prostate tissue, lined with cubic epithelium.
Figure 7. Squamous metaplasia of the acinar epithelium. Kreiberg staining. X 100
Quite often, during a hyperplastic process in the prostate gland, the acinar epithelium undergoes squamous metaplasia (Figure 7). This variant of changes in the glandular epithelium is called squamous hyperplasia, although, in our opinion, it is more correct to call it glandular hyperplasia with squamous metaplasia, since it is characterized not by the atypical development of the glandular epithelium of the prostate, but by the transformation of one type of epithelium into another. Squamous cell hyperplasia occurs in 56.8% of all cases of glandular hyperplasia.
Another variant of the metaplastic process is transitional cell metaplasia of the acinar epithelium. This type of hyperplasia is especially rare: we observed it in only 0.06% of cases of glandular hyperplasia.
With glandular hyperplasia, there may be various variants of atypical hyperplasia. The growth foci of these forms can be located in different parts of the prostate gland. The separation of atypical forms into separate groups is due to the fact that each of these options, in fact, is an optional precancer. Consequently, patients who have foci of atypical hyperplasia in the prostate gland require clinical observation and constitute a risk group for prostate cancer.
The described hyperplastic processes in the prostate gland with BPH are diffuse in nature, i.e. spread throughout the entire volume of the organ. However, there is a certain pattern in the severity of the proliferative process in its various parts.
It should be noted that the prostate gland is a fairly homogeneous organ, which normally consists only of the right and left lobes, connected by an isthmus and separated by a groove running along the posterior surface. The isthmus of the prostate gland is the area located between the entry point of the bladder neck into its base at the front, as well as the right and left ejaculatory ducts at the back. This isthmus, when hypertrophied, is considered the middle lobe of the prostate gland [4]. The connective tissue cords extending from its capsule, forming the stroma of the organ, are located between the ducts of the acini along with smooth muscle fibers. It is these fibromuscular structures that divide the gland into lobules, but their size and location are so variable that it is impractical to take them for formed anatomical structures.
In the tissue of the prostate gland, 2-3 main glands can be clearly distinguished, located in the subcapsular parts of the organ, intercalary or submucosal glands located in the intramural parts of the prostate gland, and mucous or periurethral glands.
The intensity of the proliferative process in the glandular component of the prostate varies. The acinar cells of the mucous glands proliferate most actively, the acinar cells of the intercalary (submucosal) glands proliferate less actively, and the proliferative activity of the cells of the acini of the main glands is the lowest. Naturally, this basic pattern can be subject to various changes, but they are only a deviation from the rules. Our study of sectional material from elderly people who did not suffer from urological diseases showed that the proliferative process develops both in the periurethral and subcapsular areas. In the latter case, it can even proceed with advanced intensity. However, these patients never seek urological help, since the proliferative process developing in the subcapsular zone, as a rule, is not accompanied by urination disorders, compared to those patients in whom the proliferative process develops mainly in the periurethral zone, or in the mucous glands.
The above data make it possible to justify the rejection of the term prostate adenoma, since it carries some element of depravity in the choice of treatment tactics and understanding the essence of the pathological process.
Firstly, by recognizing this term we recognize the possibility of recurrence of a benign tumor of the glandular epithelium, which recurs extremely rarely. Benign hyperplasia, in contrast, has a significant tendency to recur.
Secondly, the use of this term predetermines the absence of diffuse organ damage, which occurs with BPH.
Finally, thirdly, after removal of the adenoma, restoration of function is possible, which cannot happen with BPH.
Undoubtedly, a benign tumor of the glandular epithelium (adenoma) can develop in the prostate gland, but it is extremely rare. According to our data, based on 18,000 observations, true prostate adenoma was found in only 0.07%. In addition, it should be noted that the pathogenesis of the development of S hyperplastic and tumor processes is very different. This difference is further enhanced by the fact that the hyperplastic process in the prostate gland is of a pronounced dyshormonal nature.
The question naturally arises: why is it necessary to study morphological changes in the prostate gland during benign hyperplasia, what does knowledge of the stages of morphogenesis of this disease provide for clinical practice? The answer to this question may, to some extent, explain the causes of BPH relapses and guide urologists in choosing treatment tactics.
If, during a morphological study of prostate tissue prior to the use of any treatment method (the range of these treatment methods is currently unusually wide), proliferative centers of the I, II or III stages of development are detected, then the clinician receives a fairly good guideline for choosing a method of conservative therapy. In this situation, it is obvious that the substrate on which drugs with 5-alpha reductase activity act is preserved. On the other hand, with transurethral electroresection of the prostate gland in such patients, especially those of relatively young age, there is a very high probability of developing a relapse of BPH in the future, since this method of treatment does not have a sufficient degree of radicalism. In addition, if the biopsy specimen, in addition to the typical manifestations of BPH, reveals foci of atypical hyperplasia or foci of various variants of PIN (prostatic intraepithelial neoplasia), then thermal treatment methods should apparently be excluded, since in this case we are talking about various variants of optional precancer or cancer in place (carcinoma in situ).
The presence of proliferative centers of stages IV or V in biopsy specimens indicates that the use of conservative therapy will not lead to the desired result. The more pronounced atrophic processes are, the lower the effectiveness of drugs will be. At the same time, the presence of proliferative centers at these stages of development practically eliminates the likelihood of relapse of the disease and allows us to hope for a significant subjective improvement in the condition after surgery, since transurethral electroresection of the prostate, in addition to increasing the lumen of the urethra, will lead to decompression of a significant number of overstretched acini in the remaining part of the prostate gland.
When discussing morphological changes in prostate tissue in BPH, two questions cannot be left aside. The first of these is inflammation in the prostate tissue in this disease, the second is other processes that can imitate the changes characteristic of BPH.
According to our data, in prostate tissue with BPH, an inflammatory reaction occurs in 96.7% of cases, primarily in the form of exudative (acute) forms of inflammation.
Firstly, this is interstitial inflammation, when the inflammatory exudate spreads throughout the interstitium (pericanalicular). With this type of inflammation, isolated serous exudate practically does not occur. Most often, foci of serous inflammation are adjacent to foci of purulent inflammation, which allows them to be regarded as a progression of the inflammatory reaction. Microabscesses may form in the prostate tissue.
Secondly, the presence of purulent exudate in the lumen of the acini (intracanalicular).
Productive forms of inflammation in the prostate gland during its benign hyperplasia most often occur in the form of an interstitial process, and the inflammatory infiltrate is localized either in the stroma of the organ or periacinar. It should be noted that with various variants of productive inflammation in the prostate tissue, it is a response to some pathological processes: a change in the physicochemical properties of the prostate secretion as a result of its stagnation, proliferation of the acinar epithelium, ischemic damage to the prostate tissue and a number of other pathological processes.
Key words: prostate gland, morphology, benign hyperplasia, staging.
LITERATURE
- Structural basis of adaptation and compensation of impaired functions. / Ed. Sarkisova D.S. M. Medicine. 1987. 445 p.
- Pathological diagnosis of human tumors. / Edited by N.A. Kraevsky, A.V. Smolyannikov, D.S. Sarkisov. M. Medicine. 1993. T. 2, 686 p.
- Ham Cormack Histology / M. Mir. 1983. T. 5. 294 p.
- Sinelnikov R.D. Atlas of human anatomy. M. Medicine. 1968. T. 2. 394 p.
‹ Sample size for a population study of urological morbidity Up On the creation of a unified clinical and statistical classification of oncological urological diseases using the example of kidney tumors ›
Types of therapeutic procedures for prostate fibrosis
To improve the patient's condition, a special tube can be installed in the narrowing area in order to normalize the outflow of urine.
In case of acute urinary retention, an epicystostomy is installed - a catheter to remove urine from the bladder.
The catheter is installed by making a small incision under the navel, in the pubic area.
The procedure involves local anesthesia or general anesthesia.
The type of surgery using laser has proven itself well.
However, laser treatment of prostate fibrosis is advisable only in the initial stages of the pathological process.
The prognosis for prostate fibrosis is quite favorable, especially if you seek help in unadvanced cases.
Complications of prostate fibrosis
In the absence of adequate and timely treatment, prostate fibrosis can provoke serious negative consequences for the body.
Bladder obstruction with acute delayed deurination.
Depending on the severity, in addition to the so-called urosepsis, stagnation of urine can lead to chronic renal failure.
In addition, due to urinary retention, the acid-base balance is disturbed.
This leads to an increase in the accumulation of acids in the body, as well as an increase in the concentration of potassium in the blood, which contributes to the development of cardiac arrhythmia.
In the worst cases, when acute renal dysfunction follows, the patient is prescribed dialysis on an ongoing basis or an organ transplant.
Urolithiasis (urolithiasis)
Urolithiasis is the medical term for the presence of urinary stones (uroliths) within the urinary tract.
Such as the bladder, ureters or renal pelvis.
Urinary stones are mineral stones consisting of various crystals, usually formed from calcium oxalate.
In acute urolithiasis, complete or partial failure of kidney function occurs.
Urethrohydronephrosis
It is an abnormal expansion of the renal pelvis.
The disease is the result of bladder obstruction, which leads to disruption or destruction of kidney tissue.
If hydronephrosis becomes chronic, damage to the renal parenchyma may occur, leading to impaired renal function.
Pyelonephritis
With fibrosis, it can occur in acute or chronic form.
The disease is usually the result of a bacterial infection.
Pyelonephritis can cause many serious complications.
In some cases, simple pyelonephritis develops into purulent inflammatory disease of the pelvis.
Pathogens can then enter the bloodstream, causing the development of urosepsis.
Kidney failure
It can develop in both acute and chronic form.
The pathological process is accompanied by a violation of all kidney functions.
Kidney failure leads to the accumulation in the blood of products during protein decomposition that are toxic to the body:
- Urea. Serum and urine urea concentrations are an indicator of protein metabolism. Urea results from the breakdown of ammonia by the liver. Ammonia is excreted along with urine, as it is toxic to the body. Retention of urination due to fibrosis can lead to uremia (poisoning of the body).
- Creatinine. It is the end product of the creatine phosphate reaction, formed in the muscles, after which it is released into the blood and excreted by the kidneys. It is an important indicator of kidney function.
- Uric acid. It is formed in the body as a product of the decomposition of purine bases and is excreted from the body through urine. Serum uric acid determination is used to diagnose and monitor kidney disease. Chronic elevation of uric acid levels can lead to persistent hypertension and endothelial dysfunction.
Stages of fibrosis and symptoms
The following stages of development of prostate fibrosis are distinguished:
- There are 1-2 small foci in the gland , in 90% of cases there are no symptoms. Minor urinary disorders are rarely observed. Even with a fairly large scar, symptoms may not appear if the prostate is not in great demand (the man is not sexually active) or the lesion is located away from the urethra.
- Pathological areas become denser, grow or wrinkle (depending on the form of fibrosis), entraining and deforming organs adjacent to them. Symptoms: there is a feeling of incomplete urine output, the process itself requires more and more muscle tension, nighttime urges become more frequent, libido decreases, and erection worsens.
- The lesions are widespread throughout the gland and begin to involve the prostatic part of the urethra and bladder . Urine excretion is already significantly impaired. Symptoms: pain in the groin, urination becomes frequent, incomplete and painful, orgasm is accompanied by unpleasant sensations and a reduced amount of sperm.
- The sclerotic prostate forms a tight ring around the urethra, compresses the bladder, and the lesions block the seminal ducts flowing into the prostate . Urine and semen cease to be released. The urine accumulated in the bladder is thrown into the ureters and enters the kidneys, causing poisoning of their tissues (functional cells - nephrons - die). Symptoms: severe pain in the groin, increased body temperature, drip of urine. The last stage is characterized by weight loss, yellowing of the skin, bags under the eyes, lower back pain, dry mouth, and itchy skin.
Prostate fibrosis is often disguised as chronic prostatitis. In such cases, after a course of treatment, the tests are clear, there is no pain, but the rate of urination and sperm release is not restored, and too frequent sex leads to new inflammation.
If the focus of fibrosis has arisen, then immediate treatment is necessary, since the pathology often develops very quickly: within a couple of months the first stage will move into the second, and literally after 2 years it will already be the terminal (final) phase, life-threatening. Fibrocalcinosis progresses 2 times more actively.
Prostate fibrosis: answers to patient questions
Can prostate fibrosis cause infertility and affect conception?
Yes, fibrotic changes in prostate tissue can cause infertility. It will be possible to talk about successful conception only after full treatment.
Does Longidaza treat advanced prostate fibrosis?
Longidaza is prescribed as a prophylactic drug for patients with an increased risk of prostate hyperplasia, as well as for chronic prostatitis.
Once fibrosis has developed, the drug is no longer effective.
Prevention
Essentially, the measures to prevent prostate fibrosis are the same as for prostatitis:
- Avoid stagnant processes in the pelvis (sports, Kegel exercises, sex);
- Treat prostatitis immediately, without waiting for it to become chronic;
- Do not weaken your immune system with junk food, alcohol and nicotine.
Read more about the prevention of prostatitis.
Direct prevention of fibrosis consists of timely therapy aimed at preventing the formation of scar tissue against the background of prostate inflammation. For this purpose, simultaneously with the treatment of prostatitis, physiotherapeutic techniques, anti-inflammatory and absorbable drugs are used. As a preventative measure, these measures are much more appropriate and effective than as an early treatment for an existing lesion in the prostate.