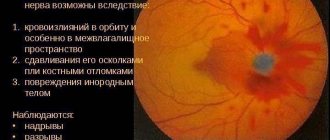
Optic nerve glioma is a common ophthalmological disease and is characterized by a pathological proliferation of astrocytes, the supporting cells of the nervous system. This disease can develop at any age, but most often occurs in children under 10 years of age. The pathology is mainly diagnosed in females. Optic nerve glioma is characterized by gradual progression and mild symptoms. Treatment is most often done with a combination of surgery and radiation therapy.
What it is?
Optic nerve glioma is a primary benign neoplasm that develops from the optic nerve trunk. This type of eye tumor accounts for 1/3 of all oncological processes in the visual organs. The pathology is characterized by an infiltrative growth pattern, does not affect the dura mater and can be located in any part of the optic nerve. Sometimes the tumor even goes deep into the skull.
The disease most often develops in children aged 5-10 years, although recently cases of glioma in adult patients over 20 years of age have increased significantly.
Often the disease occurs simultaneously with Recklinghausen's neurofibromatosis.
1.What is chiasmus?
Primary tumors arising in the area of the optic nerves and chiasm are usually of two types - gliomas and endotheliomas
. According to medical statistics, the first type of neoplasm is much more common, and is observed in most cases in children.
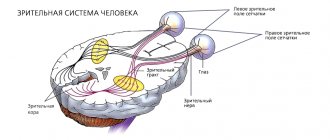
Chiasma
- a special node in which the optic nerves intersect. The chiasm is located under the hypothalamus and not far from the third ventricle of the brain. The chiasma plays a special role in transmitting signals from the visual organ to the brain. As you know, the image is perceived by the retina upside down. It is in this form that each optic nerve supplies the received information to the brain. The chiasma helps the brain correctly process the picture. Chiasma glioma can develop either independently or as a result of the growth and spread of a neoplasm of the optic nerve. The chiasm tumor itself tends to infiltrate into the hypothalamus and the cavity of the third ventricle. Some neurosurgeons are inclined to consider chiasmal glioma to be the cause of arachnoiditis and subarachnoid cysts.
A must read! Help with treatment and hospitalization!
Blood supply to the visual pathways and cortical areas of the brain
The brain tissue that conducts nerve impulses from the retina to the visual cortex, as well as the visual cortex, normally has a good supply of arterial blood almost everywhere. Several large arteries that are part of the carotid and vertebrobasilar vascular systems participate in the blood supply to these brain structures.
Using electron microscopy, similarities have been established between the capillaries of the retina, the optic disc and trunk, as well as with the capillaries of brain tissue. Unlike choriocapillaris, their endothelium does not have pores, and the endothelial cells are densely located, interlocking with each other. This suggests less permeability of the capillaries of the retina, optic nerve and brain tissue, the barrier function of which is more significant compared to the choriocapillaries.
The ophthalmic artery plays a leading role in the blood supply to the optic nerve . It arises from the internal carotid artery after this large arterial vessel, leaving the cavernous sinus, enters the subdural space of the middle cranial fossa. The ophthalmic artery, together with the optic nerve, passes through its bony canal into the retrobulbar part of the orbital cavity, where it is first located between the optic nerve and the rectus externus muscle of the eye. Then the ophthalmic artery makes an arcuate bend inward, bypassing the optic nerve from above, less often from below.
The central retinal artery departs from the arch of the ophthalmic , and in close proximity to the posterior pole of the eyeball there are several posterior short ciliary arteries (a.a. ciliaris posteriores brevis) and two long posterior ciliary arteries .
Speaking about the angioarchitecture of the optic nerve, it can be noted that it is formed by the axons of retinal ganglion cells. They are directed to the site of formation of the optic nerve head, forming a layer of nerve fibers in the retina (9th layer), which is supplied with blood mainly from the branches of its central artery. From the short posterior ciliary branches of the ophthalmic artery near the cribriform plate of the scleral canal, anastomosing branches depart, forming a ring - the vascular circle of the optic nerve (circulus vasicularis n. optici), or the arterial circle of Haller-Zinn, which is involved in the blood supply to the initial part of the optic nerve passing through the scleral canal . Small branches of the central retinal artery, extending from its fragment located as part of this nerve, also take an active part in supplying blood to the intraocular and intraorbital sections of the optic nerve.
The blood supply to other parts of the retrobulbar part of the optic nerve is provided mainly by the vascular network covering the optic nerve of the pia mater. The blood supply to the intracranial part of the optic nerve is also carried out by the vascular network of the pia mater, into which blood comes from branches extending from the internal carotid artery - the ophthalmic artery, the anterior cerebral and anterior communicating arteries.
The presence of anastomoses between the ophthalmic artery and the middle meningeal artery (a. memngea media), a branch of the external carotid artery, deserve special attention, since they may have a certain significance in the development of collateral blood supply to the eye in cases of stenosis of the internal carotid artery to the point where the ophthalmic artery originates from it.
Branches of several arteries also take part in the blood supply to the chiasm They form a powerful vascular network, which makes ischemia of the chiasm unlikely.
The main source of blood supply to the optic tract is the branches of the anterior villous artery (rami tractus optici), often arising from the internal carotid artery, proximal to its division into the anterior and middle cerebral arteries. In addition, the posterior communicating and posterior cerebral arteries participate in the blood supply to the optic tract.
The external geniculate body is supplied with blood by two arteries that are part of different vascular systems - the branches of the anterior villous artery (rami corporisgeniculatum lateralis), which belongs to the internal carotid artery, and the branches of the posterior cerebral artery (vertebrobasilar system). It is believed that the branches of the anterior villous artery provide blood supply to those neural layers of the subcortical visual center to which the lower homonymous quadrants of the retinas are projected, and the branches of the posterior cerebral artery supply blood to the projection sites of their upper homonymous quadrants. At the sites of projection onto the layers of the external geniculate body of the macula (macula) of the retina and its central fovea, blood comes from both (anterior villous and posterior cerebral) arteries.
The long optic radiation (Graziole's bundle) is supplied with blood mainly by the branches of the anterior villous and posterior cerebral arteries. Only the proximal part of the axons carrying impulses from the neurons of the subcortical visual centers, which takes part in the formation of the posterior knife of the internal capsule, is supplied with blood mainly by the branches of the middle cerebral artery.
- The blood supply to the anterior part of the optic radiation is carried out mainly by the anterior villous artery, and
- The medial occipital artery extending from the posterior cerebral artery (mainly its branches - the hemato-occipital and temporo-occipital (r. parietooccipitalis and r. occipitotemporalis), as well as the artery of the calcarine sulcus (a. calcarina) supply blood to the posterior parts of the optic radiation.
The primary and secondary visual areas of the cortex , located in the occipital lobe, are supplied with blood by the branches of the posterior cerebral artery - primarily the artery of the calcarine sulcus, the lateral and medial occipital arteries.
Only in the blood supply to the cortex of the posterior pole of the occipital lobe , onto which the macula (macula) of the retina is projected, is the participation of the branch of the middle cerebral artery penetrating here recognized. Numerous branches arise from the basilar artery and the posterior cerebral arteries that arise as a result of its division, providing blood supply to the brain stem, including the medial longitudinal fasciculus, reticular formation, subcortical centers of gaze, nuclei and proximal parts of most cranial nerves, including III, IV, V, VI, VII. In addition, the posterior cerebral arteries take part in supplying blood to certain structures of the diencephalon, including the geniculate bodies and thalamus, the posterior commissure of the brain, as well as the posterior parts of the cerebral hemispheres and the corpus callosum connecting them.
Thus, the brain structures supplied with blood from the vertebrobasilar basin include part of the visual radiation, almost the entire visual cortex and partly the cortex of the adjacent parietal and temporal lobes, as well as the occipital, midbrain and pontine oculomotor centers. The blood supply to the frontal centers of gaze, as well as the visual pathways passing through the posterior limb of the internal capsule, and, possibly, the pole of the occipital lobes (the place of projection to the visual cortex of the macula of the retina) occurs due to the branches of the middle cerebral arteries. In this regard, circulatory disorders in the vertebrobasilar system and in the middle cerebral artery can cause dysfunction of both the visual and oculomotor systems.
Causes
At the base of the optic nerve are axons surrounded by glial cells, which produce myelin, promote metabolism, and maintain the functioning of the nerve. From these cells, optic nerve glioma originates, which then grows into the surrounding membranes and spaces, moving along the nerve trunk. As the tumor grows, it increases in size and can reach the size of a chicken egg. The functions of the eye gradually become impaired, up to the complete loss of visual functions.
The exact reasons for the development of such a pathological process are still unknown. Most experts believe that glioma occurs against the background of grade I neurofibromatosis, a hereditary predisposition to cancer.
Symptoms of damage to the visual pathway
Among the signs that appear when the optic tract is damaged are the following:
- Partial loss of visual function;
- Complete blindness.
Due to the fact that the paths of nerve fibers intersect at the base of the brain, topical diagnosis becomes possible, which is based on the anatomy of the second pairs of cranial nerves:
- When the fibers on one side are completely damaged, blindness also occurs in one eye.
- If the damage is located in the central region of the chiasm, then loss of visual fields occurs on both sides (temporal region);
- Damage to the lateral parts of the chiasm leads to loss of vision on the nasal sides of both eyes.
Formation
The formation of the visual organs occurs in the fifth week of pregnancy. The optic nerve, the second of twelve pairs of cranial nerves, is formed from a section of the diencephalon along with the eyeball, resembling the stem of the optic cup.
In fact, it is a special neuron that is closely connected with the deep parts of the central nervous system.
As part of the brain, the optic nerve has no interneurons and directly carries visual information from the photoreceptors in the eye to the thalamus. The optic nerve does not have pain receptors, which changes the clinical symptoms of its diseases, for example, when it is inflamed.
During the development of the embryo, the membranes of the brain are stretched along with the nerve, which later form a special case for the nerve bundle. The structure of the sheaths of the peripheral nerve bundles differs from the sheath of the optic nerve. They are usually formed by sheets of dense connective tissue, and the lumen of the cases is isolated from the spaces of the brain.
Functions of the optic nerve
The optic nerve is the white matter of the large brain, as if brought to the periphery and connected to the brain. This substance conducts visual images from the retina, on which light rays fall, to the cerebral cortex, where the final image is formed, which a person sees.
The element is not assigned too many tasks, but all of them are of great importance in human life. Main functions of the optic nerve:
- Transfer of information from the retina to the cerebral cortex using a variety of intermediate structures.
- Lightning-fast reaction to external stimuli (loud noise, bright light, etc.). As a result, a protective reaction is triggered reflexively in the body (jumping, withdrawing the hand).
- Reverse sending of impulses from the cortical structures of the brain to the retina.
Classification of pathology
Based on the structure of the tumor tissue, optic nerve glioma can be of several types:
- astrocytoma – develops from astrocytes, occurs most often;
- ependymoma - arises from epithelial cells;
- oligodendroglioma – forms in brain tissue;
- Brain stem glioma – localized in the stem part of the central nervous system.
In adults, meningioma, a tumor growing from the cells of the arachnoid mater, can be diagnosed. There are also mixed gliomas, which consist of several types of cells. Taking into account the location of the tumor, 2 types of disease are distinguished:
- intraorbital – the tumor is localized in the cranial area;
- intracranial – the tumor is located in the orbital area;
- chiasmal glioma – pathology occurs in the area of the optic chiasm;
- intracerebral glioma – a tumor grows in the brain tissue.
In addition to true gliomas that arise as a result of the proliferation of glial tissue, gliomatosis is encountered in ophthalmology, a pathological process characterized by an increase in the size of glial cells.
Optic tract lesions
July 10, 2009
When the chiasm is completely destroyed, complete bilateral blindness occurs. But in a number of processes, damage to the optic chiasm may be limited. Thus, with tumors of the pituitary gland, expansion of the infundibuli as a result of hydrocephalus and stretching of the third ventricle, pressure can only affect the middle of the chiasm, its intersecting fibers from the inner nasal halves of the retinas of both eyes. In this case, the external or temporal fields of vision will be blind, i.e., the so-called temporal, or bitemporal, hemianopsia, which has a different name, will occur.
If only the outer corners of the chiasm are damaged (for example, with aneurysms of the carotid arteries), the temporal halves of the retinas of both eyes will be blind and a different, but already binasal hemianopsia will occur with loss of both internal fields of vision.
Much more common are the so-called homonymous, or homonymous, hemianopsias, which occur with damage to the visual pathways and centers posterior to the optic chiasm, i.e., with damage to the optic tracts, the visual thalamus, the internal capsule in its posterior section and the occipital lobe.
Starting from the optic tract, stimuli are conducted and perceived in the pathways and centers: in the right - from the right and in the left - from the left halves of the retinas of both eyes. During a break, the same hemianopsia of opposite visual fields occurs here, for example, with a lesion on the left - right-sided hemianopsia of the same name, etc.
There are some reference points for distinguishing between seemingly identical hemianopia when affected: the optic tract or subcortical visual center and the capsule (radiato optica) or cortex (fissura calcarina).
The distinguishing features for these hemianopsias will be the following:
- Tractus hemianopsia:
- simple atrophy of the optic nerves,
- hemianopic pupil reaction,
- with partial homonymous hemianopia there is often a pronounced asymmetry of visual field defects;
- central hemianopsia:
- there is no atrophy of the optic nerves,
- there is no hemianopic reaction of the pupils,
- visual field defects are usually symmetrical.
If there is asymmetry, it is not clearly expressed (E.Zh. Tron).
“Topical diagnosis of diseases of the nervous system”, A.V.Triumfov
Read further:
Pathological phenomena from the hearing aid
Pathological phenomena of the hearing aid and hearing examination are discussed in detail in the course of otolaryngology. It should be briefly mentioned that decreased hearing is designated by the term hypakusis, loss of it, that is, deafness - anakusis, or surditas, and increased perception - hyperakusis. It is always important for an otialogist and a neurologist to distinguish between hearing damage depending on pathological processes in the middle ear (tympanic membrane,…
Sensory nerve
I pair, n. olfactorius - sensory nerve. As in the visual pathway system, the cells of the first olfactory neurons are located in the periphery and not in the ganglion. Olfactory bipolar cells are scattered in the mucosa of the upper parts of the superior concha and nasal septum. The axons of these cells in the form of thin filaments (fila olfactoria) enter the cranial cavity through the lamina cribrosa ossis ethmoidalis...
N. vestibularis
The vestibular nerve, a sensory nerve, has a ganglion vestibulare Scarpae, located in the floor of the internal auditory canal. The processes of the cells of this node end in the ampoules of the semicircular canals, utriculus and sacculus. Axons entering the cranial cavity, like n. cochlearis, through porus acusticus interims, as part of the root n. vestibularis enter the brain stem at the cerebellopontine angle and end with the first neuron...
Group of nerves of the cerebellopontine angle
Spasms of the masticatory muscles are bilateral. Group of nerves of the cerebellopontine angle Nerves Nuclei, their localization Exit from the brain Exit from the skull Ganglia of sensory nerves VII Motor nucleus: pons on the border with the medulla oblongata, in the central part of the pons tegmentum In the cerebellopontine angle, above and lateral to the olive Porus acusticus internus - canalis Fallopii - foramen stylomastoideum - XIII...
N. trigeminus
Being a mixed nerve, it has motor and sensory nuclei in the brain stem. Sensitive fibers begin from the powerful Gasserian ganglion (ganglion semilunare. Gasseri), located on the anterior surface of the pyramid of the temporal bone between the layers of the dura mater. The dendrites of the cells of this node make up the sensory fibers of the trigeminal nerve, consisting of three branches: r. ophthalmicus, r. maxillaris and Mr. mandibularis. Cell axons...
Changes in the visual field with damage to the chiasm
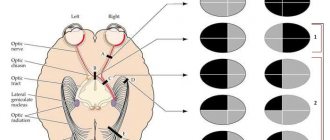
Changes in the visual field with lesions of the chiasm are very diverse. When the chiasm is damaged, heteronymous (opposite) hemianopsia may develop, which is associated with the presence of partial intersection of fibers in the chiasm and the possibility of separate damage to crossed and uncrossed bundles. Bitemporal hemianopsia occurs when the crossed fibers in the medial part of the chiasm are affected (Fig. 27).
Fig.27-32. Changes in the visual field with damage to the chiasm
Binasal hemianopsia - with damage to uncrossed fibers in the lateral parts of the chiasm on both sides by two foci (Fig. 28). If only one lesion affects the uncrossed fibers in the lateral part of the chiasm on one side, then unilateral nasal hemianopia occurs on the side of the lesion (Fig. 30). With isolated damage to crossed or uncrossed fibers of papillomacular bundles, bitemporal or binasal central scotomas occur.
If only crossed or uncrossed fibers of the papillomacular bundle of one eye are affected, then a unilateral central scotoma, temporal or nasal, occurs (Fig. 23 and 24). When the papillomacular bundles are damaged in the anterior part of the chiasm before their decussation, central non-hemianopic scotomas appear, the same as with damage to the optic nerve (Fig. 21). If the upper half of the chiasm is affected, lower hemianopsia appears (Fig. 31), and if the lower half of the chiasm is affected, upper hemianopsia appears (Fig. 32). Lower and upper hemianopsia are bilateral, as they involve the right and left halves of the visual field of both eyes.
Changes in the visual field with damage to the chiasm can be divided into developed syndromes and initial symptoms of damage to the chiasm, which can progress. Developed chiasmal syndromes include :
· complete bitemporal hemianopsia (Fig. 27),
· complete binasal hemianopsia (Fig. 28),
· blindness in one eye with temporal hemianopsia in the other eye (Fig. 29).
Blindness in one eye combined with temporal hemianopsia in the other eye occurs when half of the chiasm on the side of the blind eye is destroyed.
Initial symptoms of visual field changes may progress depending on the location of the effect and the direction of the effect on the chiasm. Let's look at these symptoms and their progression using fiber patterns in the chiasm.
1. Pressure on the chiasm from below leads to the development of partial bitemporal hemianopia with narrowing or loss of the superotemporal quadrants of the visual field (Fig. 33).
Fig.33-40. Changes in the visual field with damage to the chiasm
This occurs due to damage to the fibers coming from the inferior nasal quadrants of the retinas, which intersect closer to the inferior surface of the chiasm. Such symptoms are often caused by intrasellar tumors, including pituitary adenomas. With further upward growth of the tumor, the visual fields fall out according to Merkulov's law: first, the superior temporal quadrants drop out, then the inferotemporal, then the inferior nasal and lastly the superior nasal quadrants of the visual field of both eyes (Fig. 33 - 36). In this case, the focus affects only the medial part of the chiasm.
If the lesion under the chiasm exerts pressure from below on the entire lower surface of the chiasm and affects all crossed and uncrossed fibers coming from the lower halves of the retinas, then bilateral superior hemianopsia appears (Fig. 32). This is the second option for changes in the visual field with pressure on the chiasma from below.
The pressure from below in front is usually not strictly along the sagittal axis, but is slightly deviated to the side, therefore, in the early stage, simultaneously with the chiasm, one of the optic nerves is also affected. In this case, due to conduction disturbances along the papillomacular bundle of this nerve, a central scotoma occurs. Next, the process begins to unevenly compress the chiasm from below in front, which leads to the appearance of asymmetric (uneven) bitemporal hemianopia, and such bitemporal narrowing begins from the superotemporal quadrants. Thus, pressure on the chiasma from below anteriorly often leads to asymmetric bitemporal hemianopia in combination with a unilateral central scotoma (Fig. 37).
2. Pressure on the chiasm from above leads to the development of partial bitemporal hemianopsia with narrowing or loss of the inferotemporal quadrants (Fig. 38). This occurs due to damage to fibers from the superonasal quadrants of the retinas, which intersect closer to the superior surface of the chiasm. Similar symptoms develop if the lesion presses from above on the medial part of the chiasm.
If the focus is located somewhat posteriorly and puts pressure on the chiasm from behind from above, then the bitemporal lower quadrant hemianopia will be joined by bitemporal central scotomas (Fig. 39), which are associated with damage to the crossed fibers of the papillomacular bundles in the posterior part of the chiasm. This effect is usually caused by tumors of the pituitary gland and the bottom of the third ventricle.
Pressure on the chiasm anteriorly from above is often combined with damage to the optic nerve and causes the appearance of a unilateral scotoma in combination with partial bitemporal hemianopia (Fig. 40). Bitemporal changes also begin in the inferotemporal quadrants. Such lesions are often associated with aneurysms of the anterior cerebral and anterior communicating arteries.
3. Pressure on the chiasm from the front first causes a bitemporal narrowing of the visual field (Fig. 41).
Fig.41-50. Changes in the visual field with damage to the chiasm
Anteriorly, the chiasm is often pressured by tumors of the sphenoid sinus and meningiomas of the tubercle sella. Bitemporal narrowing occurs due to damage to part of the crossed fibers in the chiasm. With further spread of the process, all crossed fibers are affected, and complete bitemporal hemianopsia develops (Fig. 42). The spread of the process inside the chiasm may not proceed strictly along the sagittal line, but deviate to the side, which leads to additional damage to some of the uncrossed fibers in the lateral part of the chiasm. Partial bitemporal hemianopsia appears with a narrowing of the nasal half of the visual field in one eye (Fig. 43). The lesion is shifted towards the eye, in the field of vision of which there is a nasal narrowing.
If the pressure exerted by the pathological focus on the chiasm is initially slightly shifted to the side , then, having destroyed a small part of the crossed fibers of the anterior knee of the chiasm, the pathological process also affects the bundle of crossed fibers of the intracranial part of the optic nerve. A so-called junction scotoma appears, that is, a scotoma that occurs when the junction of the optic nerve and the chiasm is damaged. It is a central temporal hemianopic scotoma. Due to damage to part of the crossed fibers of the anterior knee of the chiasm, a temporal narrowing of the field of vision of the other eye appears (Fig. 44).
4. Pressure on the chiasm from behind leads to the development of partial bitemporal hemianopsia with bitemporal central scotomas (Fig. 45). This occurs due to damage to the crossed fibers of the papillomacular bundles in the posterior part of the chiasm. This picture is often caused by tumors of the pituitary stalk (infundibulum) and meningiomas of the sella diaphragm. As the process progresses, visual field defects expand to the temporal sides (Fig. 46). Central visual acuity does not fall for a long time, as it is compensated by the unchanged nasal halves of the visual field.
5. Pressure on the chiasm from the side causes damage to the uncrossed fibers in the lateral part of the chiasm. Unilateral nasal hemianopia appears on the side of the lesion (Fig. 30). If the process further spreads posteriorly, affecting the optic tract, then homonymous (tractus) hemianopsia occurs (Fig. 47). As a rule, visual field defects in the right and left eyes with such a lesion are sharply asymmetrical.
If the process from the lateral part of the chiasm spreads forward, affecting the optic nerve, then a central scotoma is added to the unilateral nasal hemianopsia on the side of the lesion (Fig. 48), and with further spread into the chiasm, temporal hemianopia also appears in the other eye due to a lesion in the chiasm of the crossed fibers and fibers of the temporal crescent of the other eye (Fig. 49).
6. The lesion in the chiasm itself often unevenly affects the fibers crossed in it, which leads to the development of asymmetric bitemporal changes in the visual field of irregular shape (Fig. 50). This is observed in inflammatory processes, gliomas of the chiasm and its traumatic tear.
Next, we will consider the pathogenesis of changes in the visual field in developed syndromes of chiasmal lesions.
1. Complete bitemporal hemianopsia occurs when all crossed fibers in the medial part of the chiasm are affected. The lesion is located medially and, as it were, divides the chiasm into the right and left halves.
2. Complete binasal hemianopsia occurs only when there are two lesions affecting all crossed fibers in the lateral parts of the chiasm on both sides. Complete binasal hemianopsia can also occur with bilateral damage to uncrossed fibers in the intracranial part of both optic nerves, which can be considered a combination of unilateral temporal hemianopsia of the right and left eyes. The features of complete bitemporal and complete binasal hemianopsia with lesions of the chiasm will be discussed in more detail in Chapter 3. It should be noted that binasal hemianopsia in clinical practice is much less common than bitemporal hemianopsia.
3. Blindness in one eye in combination with temporal hemianopia in the other eye can develop in four different variants of the localization of the pathological focus.
· The lesion in the anterior corner of the chiasm is located between the optic nerves and is adjacent to the medial surface of one of them. At the beginning of the disease - temporal hemianopsia in one eye, then temporal hemianopsia in the second eye. At the end of the disease - blindness of the initially affected eye combined with temporal hemianopia in the other eye.
Temporal hemianopsia in the initially affected eye is caused by damage to the crossed fibers in the optic nerve, to the medial side of which the pathological focus is adjacent. Temporal hemianopsia of the second eye and blindness of the initially affected eye is caused by the transition of the lesion to the chiasm, where first the crossed fibers of the second eye are affected, and then the uncrossed fibers of the initially affected eye, located in the lateral part of the chiasm.
· The lesion is adjacent to the lateral surface of one of the optic nerves . Initially, nasal hemianopsia is observed in one eye with a slight narrowing of the temporal half of the visual field of the second eye. At the end of the disease there is blindness of the eye with initially developed nasal hemianopsia and temporal hemianopsia in the second eye.
Nasal hemianopia at the onset of the disease is caused by damage to uncrossed fibers in the lateral part of the optic nerve at the chiasm. At the same time, a slight narrowing of the visual field of the second eye is caused by damage to part of the crossed fibers, which, after the cross, enter the optic nerve of the other side and form the anterior knee of the chiasm. The subsequent development of the disease is due to the transition of the lesion to the chiasm with damage to the crossed fibers of both eyes.
· A lesion in the posterior corner of the chiasm on the medial side of one of the optic tracts. At the onset of the disease, temporal hemianopia is noted in one eye, then nasal hemianopsia in the second. Collectively, homonymous hemianopsia. At the end of the disease - blindness of the eye in which nasal hemianopsia developed, combined with temporal hemianopia in the second eye.
Temporal hemianopsia of the initially affected eye occurs due to damage to the crossed fibers in the optic tract near the chiasm. The subsequent development of nasal hemianopia in the second eye is associated with damage to uncrossed fibers in the same optic tract. Blindness of this eye subsequently occurs due to the transition of the lesion to the chiasm with damage to the crossed fibers in it.
· A lesion on the lateral side of the optic tract near the chiasm . Nasal hemianopsia is observed in one eye, followed by temporal hemianopsia in the other eye. Collectively, homonymous hemianopsia. At the end of the disease there is blindness of the eye on the side of the initial nasal hemianopsia with temporal hemianopsia on the second eye.
Nasal hemianopia occurs due to damage to uncrossed fibers in the optic tract. Subsequent temporal hemianopia of the second eye is associated with damage to crossed fibers in the same optic tract. Blindness of the eye with initial nasal hemianopsia is caused by the transition of the lesion to the chiasm with damage to the crossed fibers coming from the eye with initial nasal hemianopsia.
Changes in the visual field due to chiasm pathology are discussed in more detail in Chapter 3.
Symptoms
At the initial stage of development of the disease, there are no pronounced clinical manifestations; the only manifestation is visual impairment. This symptom usually goes unnoticed in young children, and at an older age it is often mistaken for progressive myopia. As the tumor grows, the symptoms become more pronounced, the following signs appear:
- exophthalmos (protrusion of the eyeball);
- strabismus;
- keratitis;
- scotoma;
- restriction of eyeball movement;
- inability to close eyelids;
- drying of the cornea;
- keratitis, corneal ulcers.
Optic nerve glioma is accompanied by progressive and irreversible vision loss. Cysts often form in the tumor cavity, and an increase in the degree of hyperopia is also observed. If a glioma of the chiasm and optic nerves is formed, the following symptoms are observed:
- decreased visual acuity in both eyes;
- developmental delay;
- nystagmus;
- endocrinological disorders.
You can also suspect the development of a benign neoplasm in the visual structures based on the following signs:
- sleep disturbance;
- nausea, vomiting;
- frequent migraines;
- lethargy;
- loss of appetite;
- pain behind the eyeballs;
- memory impairment;
- excessively protruding veins on the head.
When a tumor develops intracerebrally, symptoms depend on which part of the brain is affected. The most common types of ataxia, hydrocephalus and sensory disturbances are observed.
Lesions of the lateral geniculate body
The cytoarchitecture of the LCT is determined by its six-layer lamellar structure, which is found only in higher mammals and primates. The anatomy and physiology of LCT are presented in sufficient detail in the literature. LCT is a paired formation of the visual pathways of the brain related to the metathalamus. The dimensions of the LCT are 8.5 x 5 mm. Each LCT contains two main nuclei: dorsal (upper) and ventral (lower). The main dorsal nucleus (nucleus dorsalis) consists of four layers of concentrically located small nerve cells (parvocellular layers - 3, 4, 5 and 6 P cells). The ventral nucleus (nucleus ventralis) consists of two layers of large nerve cells (magnocellular layers—1 and 2, M-cells). In each LCT, both crossed and uncrossed visual fibers from the retinas of both eyes end in strictly certain layers. Through the nerve fibers of the optic radiation (tractus geniculooccipitalis), the LCT is connected to the visual area of the cerebral cortex (Brodmann area 17). The main functional unit of the LCT (as well as the retina) is the receptive field. The receptive field is a set of neurons at a lower level that are functionally connected to one neuron at the next higher level. The receptive fields of LCT neurons have a concentric shape and are similar to the receptive fields of retinal ganglion cells. LCT neurons are divided into two antagonistic classes with on- and off-centers. Neurons with an op-center, when stimulated, increase the activity of the receptive field, and neurons with an off-center suppress it. The structure and function of the receptive fields of the LCT reflect the properties of the receptive fields of the retina. However, LCT neurons, compared to retinal neurons, have a more subtle mechanism of contrast sensitivity due to the proximity of the inhibitory and excitatory regions of the receptive field. One of the important functions of LCT is to provide binocular vision. With pathological processes in one LCT, the visual functions of both eyes are disrupted. In the study of the functions of the LCT, experimental studies on animals (mainly on monkeys) are essential, allowing one to obtain data on the factors and mechanisms of interaction of neurons of the visual pathway (including the LCT) with retinal ganglion cells. One of the significant factors of this interaction is the orthograde and retrograde flow of axoplasm (cerebrospinal fluid), which ensures the vital activity of the central nervous system, including the above-mentioned parts of the visual pathway. In this current there are slow and fast phases. The slow phase of fluid movement is 0.5-2 mm per day, the fast phase - 20-400 mm per day. A. E. Hendrickson, using electron microscopy, studied axoplasmic transport in the visual pathway of monkeys. 3 days after injection of tritium-labeled leucine into the vitreous body of a monkey's eye. Greater radioactivity was detected in the synaptic terminals of the axons of retinal ganglion cells in the LCT on the opposite (contralateral) side. This demonstrated the rapid phase of axoplasmic transport. The slow phase of the axoplasmic current from the eye reaches the LCT only 27-30 days after the introduction of the radioactive substance into the vitreous body. Impaired axoplasmic transport is observed clinically in ischemic diseases of the retina and optic nerve and intracranial hypertension. Increased intraocular pressure (IOP) in monkeys reduces protein synthesis in retinal ganglion cells and reduces axoplasmic transport of nutrients. The experiment showed that axoplasmic transport occurs not only in the proximal direction (from the periphery to the center), but axoplasmic fluid flow can also occur in the opposite - retrograde - direction. The system of bidirectional axoplasmic transport promotes the most rational use of nutrients by retinal and LCT neurons. D. Fritzpatric et al. studied the lamellar structure of the LCT in monkeys (macaques) using the method of retrograde injections of a contrast agent. It was noted that neurons of the 6th layer of the LCT are connected predominantly with the parvocellular layers of retinal ganglion cells. Vickers et al. studied neurochemical changes in LCT nerve cells in monkeys when glaucoma was modeled by exposure to laser radiation on the trabecular meshwork of the anterior chamber angle. In connection with an increase in IOP, presynaptic and postsynaptic pathological changes in the magnocellular and parvocellular layers of LCT cells were identified. As shown by studies on monkeys by AJ Weber et al., larger retinal ganglion cells, ending with their axons in the magnocellular layer of LCT cells (M-path), are especially sensitive to ocular hypertension. Numerous dwarf retinal ganglion cells ending in the parvocellular layers of the LCT (P-ggut) are less susceptible to the adverse effects of increased IOP. AJ White et al. studied the receptive fields of conicellular, parvocellular and magnocellular nerve cells in the LCT of monkeys. Methods for studying contrast sensitivity were used. It was noted that the diameter of the receptive field in all classes of LCT neurons decreases with increasing distance from the projection of the macular area. W. M. Usrey et al. Studies on cats have shown that retinal ganglion cells and LCT signaling neurons have a very similar structure of receptive field centers. The strongest functional connections between the retina and the LCT are formed when the surfaces of the receptive fields of the retinal ganglion cells and the LCT overlap each other by at least 50% of their area. The authors suggested that LCT neurons often receive their energy supply from afferent (centrifugal) nerve fibers emanating from retinal ganglion cells with the above relative positions of receptive field centers. The close connection between the retina and LCT is confirmed by studies on kittens and young monkeys raised with the eyelids of one eye sutured for 3 months after birth. The activity of LCT nerve cells, which receive impulses from the deprived eye, was reduced and significant histological changes were found in them: neurons were reduced in size by 30% and contained shriveled nuclei. Eye deprivation for a shorter period (2 months) caused less pronounced changes in the LCT nerve cells. Ocular deprivation in adult cats did not lead to changes in LCT neurons. Consequently, during the period of maturation of the visual system, eye deprivation affects not only the neural structures of the retina, but also the neurons of the LCT. Similar changes were observed in people with amblyopia in one eye. Thus, the results of experimental studies indicate a close functional relationship between LCT neurons and retinal ganglion cells. Violation of this relationship (with an increase in IOP, eye deprivation) leads to functional and morphological changes in retinal ganglion cells and LCT. This should be taken into account when studying the pathogenesis and clinic of lesions of the retina and lateral geniculate body. Isolated lesions of the LCT are rarely observed in the clinic, however, the anatomical proximity of the thalamus and the common sources of blood supply to both brain structures determine their frequent joint lesions in vascular disorders and tumors in the area of the visual pathway of the brain. Impaired function of the LCT can be caused by vascular pathology (vasospasm, hemorrhage, infarction of the LCT, congenital vascular changes of the LCT), as well as its damage in multiple sclerosis, and the development of a brain tumor (usually astrocytoma). Retrochiasmal lesions of the optic pathway are characterized by the appearance in the field of view of both eyes of varying degrees of severity of homonymous hemianopia and a decrease in visual acuity depending on the involvement of the optic fibers of the papillomacular bundle in the pathological process. JL Smith analyzed 100 cases of hemianopia, of which 39 cases involved the occipital lobe of the brain, 33 involved the parietal lobe, and 24 involved the temporal lobe of the brain. In 4 patients, disorders were localized in the optic tract and LCT.
{module direct4}
Retrospectively analyzing the causes and frequency of retrochiasmatic lesions in various parts of the visual pathway in 140 patients, T. Fujino et al. give the following results. The main cause of damage to the visual pathway above the chiasm is impaired blood circulation in the vessels of the brain (98 patients), in second place are brain tumors (25 patients) and in third place are inflammatory, demyelinating and degenerative processes (17 patients). Most often, the cortical part of the visual tract in the occipital lobe is affected (51%), in second place is the optic radiance (29%), and in third place is the posterior part of the optic tract and LCT (21%). Clinical manifestations of optic pathway pathology in the area of LCT with ophthalmological symptoms are still not sufficiently reflected in the literature. The main symptom of impaired visual function with damage to the LCT is the appearance of homonymous hemianopsia, i.e. the formation of characteristic defects in the visual field of both eyes - loss of half of the visual field. Descriptions of isolated patients with LCT lesions of various etiologies with quadrant hemianopia are provided. S. N. Gunderson and W. F. Hoyt first described in two patients with LCT lesions the appearance of inappropriate (incongruent) scotomas in the field of view of both eyes along the horizontal meridian. These are the so-called geniculate (geniculate) hemianopsias. In one of the patients observed by the authors, damage to the LCT occurred due to a congenital defect of the vascular system of the brain (arteriovenous malformation), in another patient - due to the development of a tumor (astrocytoma) of the brain. Later, WF Hoyt observed two more patients with geniculate hemianopsia - inconfluent defects in the visual field caused by vascular disorders affecting the LCT. Such characteristic homonymous visual field defects correlated with damage to the optic fibers of the retina. Typical geniculate hemianopsias appear only in the early stages of brain tumor development involving the LCT. As the tumor grows, geniculate hemianopsia usually turns into complete hemianopsia after 5-6 months. L. Frisen et al. It was possible to identify characteristic symptoms that may allow clinicians to diagnose vascular lesions of the LCT. It should be taken into account that each LCT receives blood supply from two sources: from the anterior choroidal artery (a branch of the anterior cerebral artery) and from the lateral choroidal artery (a branch of the posterior cerebral artery). In LCT infarctions, due to occlusion of one of these choroidal arteries, characteristic strictly congruent hemianoptic scotomas appear. Subsequently, such patients develop partial atrophy of the optic nerves with “hemianopsic” atrophy of the retinal nerve fibers. Progressive pathological changes in the posterior parts of the optic tract and LCT only after a significant period of time (weeks and months) lead to the appearance of typical symptoms of optic nerve atrophy. Shacklett et al. reported the appearance of characteristic wedge-shaped prolapses in the visual field of two sick women aged 52 years. One patient had a LCT infarction, confirmed by computed tomography and cerebral angiography, and the other had a cerebral astrocytoma. Wada et al. described a patient in whom, as a result of a fresh LCT infarction, an incongruent homonymous hemianoptic scotoma appeared in the visual field. Homonymous hemianopsia can be combined with manifestations of the thalamic Dejerine-Roussy syndrome, in which hemianesthesia and hemyalgia develop on the opposite side of the body. With retrochiasmal lesions of the visual pathway, the diagnostic information value of the pattern of visual evoked potentials (VEP) is lower than the data obtained from perimetry. Homonymous defects in the visual field cause a decrease in the bioelectrical activity of neurons in the cortical sections of the visual analyzer. In this regard, when stimulating the entire visual field, VEP asymmetry is recorded. If homonymous visual field defects include the macular region, then upon stimulation of the seeing half of the visual field, the VEP are changed, acquiring a form characteristic of absolute scotomas located in the central visual field. When the functions of the macular area are preserved, the VEP does not change, remaining normal. Damage to the optic nerve and other parts of the visual pathway, including the LCT, can occur as a result of multiple sclerosis. One of the characteristic manifestations of multiple sclerosis is demyelination of the nerve fibers of the visual pathway. O. A. Khondkarian, I. A. Zavali-shin, O. M. Nevskaya identify the ocular variant of the cerebral form of multiple sclerosis. In multiple sclerosis, plaques are localized around the ventricles of the brain and the likelihood of damage to the nerve structures of the visual pathway is very high. N. Evangelon et al. conducted histological studies of the peripheral part of the visual pathway in patients who died from multiple sclerosis. It turned out that axon loss in multiple sclerosis is 45% greater than in the control group. In multiple sclerosis, small neurons in the parvocellular layers of the LCT are more damaged than large neurons in the magnocellular layers.
A case of blindness as a consequence of acute damage to the visual pathways in the chiasm zone due to a pituitary tumor
AV Kuroedov, EN Aref`eva, NM Solnov, ZP Kushim In the article the case of occurrence of blindness of the patient with the microadenoma of the hypophysis is described, which is mostly connected with rare complication of gamma-therapy.
The pituitary gland is an endocrine gland that produces a number of hormones and is a small organ shaped like an ellipse and located under the base of the brain in the sella turcica of the main bone [2,4]. The average dimensions of the pituitary gland in the anteroposterior direction are 10 mm, in the dorsoventral direction – 6 mm and in the transverse direction – 13 mm. Weight – on average 0.57 g [5.6]. The anatomical structure of the pituitary gland is represented by three lobes, different in their development, structure and functions: anterior (glandular), intermediate and posterior (nervous). The anterior lobe develops from the ectoderm of the pharynx, which grows in the form of a long protrusion towards the neural lobe. The apex of this protrusion at the point of closure with the posterior lobe forms the intermediate lobe. The neural lobe develops from a protrusion of the interstitial medulla in the area of the gray tuberosity and subsequently becomes almost entirely surrounded by the anterior lobe (Fig. 1). To understand the expected clinical picture of chiasmal diseases in tumors of the pituitary gland, the hormones of its anterior lobe are important. These are growth hormone, two gonadotropic hormones, thyroid-stimulating and adrenotropic hormones. Growth hormone regulates the growth of the body, gonadotropic hormones stimulate spermatogenesis and the development of follicles in the ovaries, thyroid-stimulating and adrenotropic hormones regulate the activity of the thyroid gland and adrenal glands [5,6,7]. In Russian literature, the first monograph on pituitary tumors dates back to 1910. This is the work of N.R. Botvinnik, E.R. Hesse and E.A. Giese. In it, the authors reviewed the ophthalmological and neurological symptoms and radiological signs of pituitary tumors [3]. The modern classification of pituitary tumors proposed by E. Horvath and K. Kovacs (1995), based on more than 1,700 histological and other types of studies, indicates that the most common are somatotropinomas (13–15%), prolactinomas (25–28%), null cell adenomas (13–15%) and oncocytomas (10–12%) [1]. Endocrine symptoms make it possible to differentiate chiasmal diseases caused specifically by pituitary tumors, as opposed to chiasmal diseases of other origins. Of the total number of endocrine disorders caused by pituitary tumors, the most significant can be identified: acromegaly, adiposogenital dystrophy, Itsenko-Cushing's disease, diabetes insipidus, obesity and sexual dysfunction [5]. The clinical picture of pituitary tumors in the majority of patients (79%) manifests itself typically, mainly expressed by three characteristic symptom complexes (Hirsch triad). Firstly, this is a neuro-ophthalmological symptom complex - primary atrophy of the optic nerve and changes in the visual field, often in the form of bitemporal hemianopsia. Secondly, the endocrine-metabolic symptom complex - acromegaly, adipose-genital dystrophy, sexual function and metabolic disorders. Thirdly, the radiological symptom complex is the presence of a balloon-shaped expansion of the lumen of the sella turcica with an increase in its size and a uniform arched descent of the bottom of the sella. In this case, the back of the sella straightens and becomes thinner, and the size of the entrance to the sella increases relatively little [3]. Currently, three types of treatment for pituitary adenoma are used: surgical, radiotherapy and medication. Irradiation of the pituitary gland is the most common treatment method and produces good results in most cases. The effectiveness of telegammatherapy reaches 75%. The standard course dose of radiation is usually 45–50 Gray, prescribed over 4 fields and carried out for 4–5 weeks. Surgical treatment is performed in the presence of visual impairment and severe neurological disorders (for example, severe headaches) [8]. Drug treatment includes the use of selective D2 receptor agonists (Bromocriptine, Parlodel). Materials and methods Under our supervision is patient M., 67 years old, who was admitted to the endocrinology department with complaints of complete blindness and a diagnosis of pituitary microadenoma (somatotropinoma), hormonally active. Acromegaly: hypertrophic stage, stable phase, benign course. Multinodular euthyroid goiter, stage II. High myopia in both eyes. From the anamnesis it follows that in the late 80s the patient began to be bothered by headaches localized in the frontal and temporal regions, facial features became larger, dental diastasis appeared in both jaws, the size of her hands and shoe size began to increase (from 36 to 39– 40) (Fig. 2,3). The first visit to an endocrinologist was recorded in the medical documentation in 2002, when a pituitary adenoma was diagnosed. At this time, MRI of the brain and ultrasound of the thyroid gland were performed (Fig. 4,5). On MRI (October 31, 2002): the pituitary gland is enlarged in size to 9x15 mm. In its right parts, a zone of increased magnetic resonance signal is determined on T2 images measuring 6x4 mm without signs of volumetric effects. Thus, in October 2002, a pituitary adenoma was objectively diagnosed. Ultrasound of the thyroid gland from November 2003: smooth contours, isthmus 4 mm, right lobe 43x22x27 mm, left lobe 43x20x24 mm. In the lower pole of the right lobe there is a cystic nodule 20x23 mm, above it a node of mildly reduced echogenicity is 12x8 mm, and in different parts of the left lobe there are at least 5 small hypoechoic nodules with a diameter of 3-6 mm. Ophthalmological status from 08.10.2002: vis OD=0.01 csph – 9.5D cyl – 1.25D ax 60°=0.7 vis OS=0.01 csph – 9.0D cyl – 0.75D ax 60°= 0.7/0.6 ONH OU with clear contours, without stagnation phenomena, bilateral myopic staphylomas, more pronounced in the temporal halves, arteries are clearly sclerotic, tortuous, narrowed. The veins are slightly dilated. There are no focal symptoms. IOP level is 17 and 18 mm Hg. Fields of view unchanged. Taking into account the dynamics of the development of the disease and the clinical picture of the disease, the patient was recommended an outpatient course of radiation therapy, which she underwent from November 18, 2002 for 4 weeks, including remote gamma therapy from three fields, 5x5 cm and with convergence of 1 field at 27 degrees . SOD 60 Gr. After irradiation, the headaches became less for some time, but they reappeared in February–March. From October 2002 to October 2003, the patient was under dynamic outpatient supervision by an endocrinologist and ophthalmologist. In dynamics, a study of somatotropic hormone (GH) was carried out on October 22, 2002 - 14.3 IU/l; 04/09/2003 – 45.5 IU/l; 10/16/2003 – 49.8 IU/l; 10/22/2003 – 27.5 IU/l (N 0.2–13) and computed tomography (05/31/2003) – without negative dynamics. I took parlodel 15 mg/day. No negative dynamics in the ophthalmological status were also noted. In mid-October (October 16–30, 2003), the patient was hospitalized in the endocrinology department for planned treatment. A repeat MRI of the brain on October 27, 2003 did not reveal any signs of negative dynamics. The ophthalmological status remained the same. But already from mid-November 2003, the patient began to notice deterioration in vision, expressed in a decrease in central visual acuity and a concentric narrowing of the visual fields. During the control examination on November 13, 2003, visual acuity with the previous correction was already 0.2/0.1, and a concentric narrowing of the visual fields was noted (Fig. 6). This was accompanied by increased headaches. A decision was made to hospitalize him. In dynamics: acuity from November 25, 2003 – 0.1/0, December 4, 2003 – 0/0. In this case, pallor of the optic disc OU without signs of edema was noted. On December 4, 2003, a repeat NMR study was carried out (Fig. 7,8,9). A series of tomograms obtained before and after intravenous administration of 10 ml of the Omniscan preparation revealed a slight thickening of the chiasm, accumulation of a contrast agent in its left half, and an increase in signals along the optic tracts symmetrically on both sides. The size of the pituitary gland does not appear to be enlarged, the chiasmal cisterns are clearly visible, the intraorbital sections of the optic nerves are not changed, the ventricular system is not dilated or deformed, and the paranasal sinuses are transparent. In conclusion, it was suggested that the damage to the chiasm and optic tracts was caused by radiation. At the same time, the patient received consultations from a number of leading specialists (radiologists, neuro-ophthalmologists). There were no signs of continued growth of the pituitary tumor. The clinical and radiological picture of radiation damage to the optic nerves and optic tracts after radiation therapy for a pituitary tumor is determined. A course of treatment was prescribed, including hormonal (general and local) therapy, vascular and destructive therapy, hyperbaric oxygen therapy, hirudotherapy, and vitamin therapy. For a diagnostic search and to clarify the objective state of the visual pathways, electrophysiological research methods were used. Studies of evoked potentials of the cerebral cortex dated December 25, 2003 revealed pronounced signs of impaired conduction along the visual pathway of both eyes. Changes in the outer layers of the retina were also diagnosed. Phosphene is not called. Unfortunately, at present it is not possible to present an objective picture of the state of the optic disc (photo or retinotomograph) due to the fact that due to amaurosis the patient cannot fix her gaze on one supposed point even for 3 seconds. Conclusion Apparently, in this observational case we encountered a rare complication of gamma therapy, which manifested itself in the form of radiation damage to the optic nerves and visual tracts. Edema due to radiation injury caused compression of the chiasm, which in turn led to primary atrophy of the optic nerves, in contrast to secondary atrophy, which is caused after congestion. At the same time, the assumption that descending atrophy has developed seems debatable due to such a rapid development of blindness. In addition, we cannot document the moment of onset of bitemporal hemianopsia, which appears so often and precedes a decrease in central visual acuity. The current state of the diagnostic search makes it possible to differentiate this complication from the continued upward growth of a pituitary tumor, when the tumor compresses the fibers of the medial part of the chiasm at its lower surface. Visual acuity remains normal, but changes in the visual field are detected, expressed in bitemporal hemianopsia [8]. The prescribed treatment has not yet led to any positive dynamics in the patient’s ophthalmic status.
References 1. M.I. Balabolkin // Endocrinology. – M. – Universum publishing. – 1998. – 582 p. 2. M.P. Krasnov, M.G. Margolis // Outpatient Ophthalmology. – M. – Medicine. – 1969. – 360 p. 3. A.Ya. Samoilov, V.M. Pantieleva, O.N. Sokolova et al. // Ophthalmological symptoms of brain tumors. – M. – Medgiz. – 1959. – 228 p. 4. O.N. Sokolova, Yu.N. Volynskaya // Tumors of the optic nerve and chiasm. – M. – Medicine. – 1975. – 224 p. 5. E.Zh. Tron // Diseases of the visual pathway. – L. – Medgiz. – 1955. – 388 p. 6. E.Zh. Tron // Diseases of the visual pathway in brain tumors. – Sat. “Question. neuroophthalmol.” – L. – 1955. – P. 40–96. 7. E.Zh. Tron // Eye and neurosurgical pathology. – L. – Medicine, Leningrad. department – 1966. – 491 p. 8. L. Bakay // The results of 300 pituitary adenoma operations.– J. of Neurosurgery.– 1950.– vol. 7.– P. 240.
Diagnostics
Optic nerve glioma develops slowly and characteristic symptoms usually appear in advanced stages, when it is no longer possible to regain lost vision. Therefore, it is important to detect and fix the problem in a timely manner. You should consult a doctor for diagnosis as soon as a deterioration in the quality of vision becomes noticeable.
Read in a separate article: Papilledema: symptoms, causes and treatment
Based on the complaints, the specialist will be able to predict the development of the pathological process. However, in order to make an accurate diagnosis, this is not enough; a number of instrumental studies are necessary:
- ophthalmoscopy;
- visometry;
- perimetry;
- biomicroscopy;
- ultrasonography;
- radiography.
The most accurate methods for diagnosing benign formations of the optic nerve are CT and MRI. Tomography allows you to verify the presence of a tumor, as well as determine its exact location, structure and degree of activity of spread, germination into the skull area. A tissue biopsy and subsequent histological examination are also required.
Such diagnostic measures are necessary to determine the nature of the neoplasm.
Early symptoms of papilledema that may help in diagnosis
Diagnosis of the pathological process may include:
- taking anamnesis;
- determination of visual boundaries;
- ophthalmoscopy;
- Ultrasound;
- MRI or CT;
- optical coherence tomography;
- fundus fluorescein angiography (FAGD);
- lumbar puncture.
When collecting anamnesis, the specialist finds out the symptoms of the pathology, the probable causes of its occurrence, and conducts primary tests.
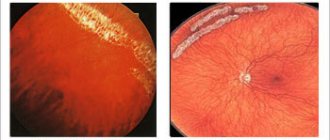
During ophthalmoscopy, the optic disc, retina, vessels of the visual organs, as well as the pupil and fundus are examined. The examination helps to identify thickened areas, tortuosity of veins, disc swelling, hemorrhages, etc.
During FAHD, photographs are taken of the ocular vessels that have acquired a specific shade due to the injected fluorescein. The procedure is carried out to diagnose damage to the retina and fundus of the eye, visualize the microcirculation of the organ of vision.
Ultrasound helps determine pseudoedema of the nerve. MRI or CT is performed for congestive processes in the fundus.
As already described above, the first method used to identify pathology is ophthalmoscopy. However, the symptoms that can be detected with this test do not allow a definitive diagnosis.
Deterioration of vision, lack of pupillary response to light, narrowing of the blood vessels in the eye are signs of many eye diseases, for example, peripheral cataracts. In this regard, many different methods are used to diagnose atrophy:
- visometry - testing visual acuity;
- perimetry - a study that allows you to measure the field of vision;
- color perception study;
- CT scan;
- examination of the fundus through the pupil after instillation of mydriatics;
- radiography of the skull;
- fluorescein angiography - examination of the fundus and retinal vessels;
- MRI of the brain and orbit.
Laboratory tests are also carried out. The patient donates blood and urine for analysis. Tests are prescribed for syphilis, borreliosis and to determine other non-ophthalmological diseases.
Treatment options
It is impossible to get rid of optic nerve glioma using a conservative method. Treatment of the tumor can be carried out in the following ways.
Radiation therapy
One of the most effective methods of treating optic nerve glioma, which is carried out in the early stages. Indications for irradiation are small tumor sizes and intraorbital location. Not only the tumor, but also 2 cm around it is exposed to irradiation. In most cases, the prognosis is positive, the glioma stops growing, and vision is preserved.
However, it should be borne in mind that radiation therapy can cause various side effects, especially in childhood, when the body is still fragile.
Surgical intervention
The operation is performed if the patient’s vision is rapidly deteriorating or there is a high risk of complications. The complexity of surgery depends on the volume of the tumor:
- Small tumors are removed through orbitotomy. Sometimes resection of the damaged portion of the optic nerve is performed.
- Gliomas that have reached the scleral ring and continue to grow are eliminated by enucleation of the eyeball. To achieve a greater effect, enucleation is performed followed by the formation of a supporting stump.
In cases where the tumor grows into the cranial cavity, the decision about the possibility of surgery is made by a neurosurgeon.
Chemotherapy
It is performed after surgery to destroy any remaining cancer cells. The patient is prescribed a course of medications, in particular, glucocorticosteroids should be taken, which help reduce swelling of the skull. This is especially necessary if the cancer spreads to other parts of the brain.
However, due to the fact that such medications stop cell growth, this negatively affects the condition of the entire body. Therefore, doctors most often combine chemotherapy with radiation.
This method of treatment has many side effects, as a result of which it is not recommended in childhood.
4. Treatment of the disease
Treatment of glioma
is mainly carried out surgically. If the size and location of the tumor allows, orbitotomy
with preservation of the eyeball. In cases where the chiasmal glioma has significantly infiltrated into the optic nerve, it is usually partially removed to reduce the risk of vision loss.
Along with surgical treatment of glioma, radiotherapy and chemotherapy
, both as a primary and an auxiliary method of treatment - it depends on the patient’s condition, character
Forecast
Optic nerve glioma is a dangerous disease that, if not treated promptly, leads to complete loss of vision in one or both eyes. The prognosis for this disease depends on the extent of the cancer process and the size of the tumor. If the pathology was detected at the initial stage of development, then with proper therapy, vision can be at least partially preserved. If the problem is detected in the later stages, the prognosis is extremely unfavorable.
Author of the article: Kvasha Anastasia Pavlovna, specialist for the website glazalik.ru Share your experience and opinion in the comments.
If you find an error, please select a piece of text and press Ctrl+Enter.
Types and forms of optic nerve atrophy
Among the key processes leading to increased pressure inside the skull:
- growing tumors in the local area;
- swelling of the central nervous system organ;
- inflammatory process in tissues, brain membranes;
- traumatic brain injury;
- pathological processes associated with blood;
- allergic reactions;
- increased blood pressure in the body;
- kidney ailments.
With atrophy of the optic nerve, its fibers are completely or partially destroyed. They are subsequently replaced by connective tissue. The death of the fibers causes the light signals received by the retina to be converted into electrical signals that are transmitted to the brain.
For the brain and eyes, this process is pathological and very dangerous. Against this background, various disorders develop, including decreased visual acuity and narrowing of its fields. Optic nerve atrophy is quite rare in practice, although even the most minor eye injuries can provoke its onset.
Optic nerve atrophy is one of the symptoms of various eye diseases or a stage in the development of any disease. There are many reasons that can lead to this pathology. Among the ophthalmological diseases that can provoke atrophic changes in the optic nerve are the following ailments:
- glaucoma;
- retinal pigmentary dystrophy;
- myopia;
- uveitis;
- retinitis;
- optic neuritis,
- damage to the central artery of the retina.
Atrophy can also be associated with tumors and diseases of the orbit: optic nerve glioma, neuroma, orbital cancer, meningioma, osteosarcoma and others. All kinds of diseases of the brain and central nervous system in some cases lead to atrophic processes in the eyes, affecting primarily the visual nerves. Such diseases include:
- multiple sclerosis;
- pituitary tumors;
- meningitis;
- brain abscess;
- encephalitis;
- traumatic brain injuries;
- damage to the facial skeleton with injury to the optic nerve.
Quite often, atrophy of the optic nerve occurs due to hypertension, atherosclerosis, diabetes mellitus. The optic nerve can begin to atrophy during fasting, severe poisoning with alcohol, nicotine and medications.
A sudden large loss of blood can also trigger atrophic changes in the optic nerve. Its degeneration also develops against the background of systemic lupus erythematosus and such rare pathologies as Behcet's, Horton's, and Takayasu's diseases.
Sometimes atrophy is caused by complications of bacterial infections, for example, syphilis and tuberculosis, parasitic and viral pathologies: influenza, ARVI, measles. All these reasons relate to acquired atrophy. It can be congenital and occurs with micro-, macro- and acrocephaly and against the background of various hereditary syndromes.
This pathological condition can be congenital or acquired. Acquired atrophy is divided into descending and ascending. In the first case, the fibers of the optic nerve are directly affected. In the second, the cells of the retina come under attack. According to another classification, acquired atrophy can be:
- Primary. It is also called a simple form of atrophy, in which the optic disc becomes pale, but has clear boundaries. The vessels in the retina with this type of pathology narrow.
- Secondary, which develops due to inflammation of the optic nerve or its stagnation. The boundaries of the disc become unclear.
- Glaucomatous, accompanied by increased intraocular pressure.
Based on the extent of damage to the optic nerve fibers, atrophy is divided into partial and complete. The partial (initial) form manifests itself in severe deterioration of vision, which cannot be corrected with contact lenses and glasses.
At this stage, the remaining visual functions can be preserved, but color perception will be severely impaired. Complete atrophy is damage to the entire optic nerve, in which a person can no longer see anything with the affected eye.
Optic nerve atrophy manifests itself in a stationary form (does not develop, but remains at the same level) and progressive. With stationary atrophy, visual functions remain in a stable state.
The progressive form is accompanied by a rapid decrease in visual acuity. Another classification divides atrophy into unilateral and bilateral, that is, with damage to one or both organs of vision.
The first and main symptom that manifests itself in any form of optic nerve atrophy is blurred vision. However, it cannot be corrected. This is a sign by which the atrophic process can be distinguished from ametropia - a change in the ability of the human eye to correctly refract light rays.
Vision can deteriorate gradually and rapidly. It depends on the form in which atrophic changes occur. In some cases, visual functions decrease within 3-4 months, sometimes a person becomes completely blind in one or both eyes within a few days.
In addition to the general decrease in visual acuity, its fields are narrowed. The patient almost completely loses lateral vision, which leads to the development of the so-called “tunnel” type of perception of the surrounding reality, when a person sees everything as if through a pipe.
Another common sign of optic nerve atrophy is the appearance of scotomas - dark or blind areas that appear in the field of vision. By the location of the scotomas, you can determine which fibers of the nerve or retina are damaged the most.
If spots appear right in front of the eyes, then the nerve fibers located closer to the central part of the retina or directly in it are affected. Color vision disorder becomes another problem that a person faces with atrophy. Most often, the perception of green and red hues is impaired, rarely - the blue-yellow spectrum.
All these symptoms are signs of the primary form, that is, its initial stage. The patient himself can notice them. Symptoms of secondary atrophy are visible only during examination.
As soon as a person consults a doctor with symptoms such as decreased visual acuity and narrowing of its fields, the doctor conducts an examination. One of the main methods is ophthalmoscopy - examination of the fundus of the eye using special instruments and devices. During ophthalmoscopy, the following signs of optic nerve atrophy are revealed:
- vasoconstriction;
- varicose veins;
- disc blanching;
- decreased pupil reaction to light.
It is impossible to restore fibers that have already been destroyed. Treatment helps stop atrophy and save those fibers that are still functioning. There are three ways to combat this pathology:
- conservative;
- therapeutic;
- surgical.
With conservative treatment, the patient is prescribed vasoconstrictors and medications, the actions of which are aimed at normalizing the blood supply to the optic nerve. The doctor also prescribes anticoagulants, which inhibit blood clotting activity.
Physiotherapeutic treatment involves prescribing:
- Electrical stimulation is a procedure in which a special electrode is placed behind the eyeball and electrical impulses are transmitted to the optic nerve.
- Magnetic stimulation, which creates a magnetic field that affects nerve fibers. Thanks to this procedure, the tissues of the optic nerve are saturated with oxygen, as blood supply improves.
- Laser stimulation: the optic nerve is exposed to a special emitter through the pupil or cornea.
- Ultrasound therapy, which allows you to activate metabolic processes in the eye. This treatment method is not always effective. It does not help if the atrophy is caused by encephalitis or tuberculous meningitis.
- Electrophoresis. This is a procedure characterized by the effect of low-intensity current on the tissue of the eyeball. This method of treatment leads to vasodilation and improves metabolism.
- Oxygen therapy. Atrophy is a lack of nutrition of cells and tissues, therefore one of the main directions of therapy is the saturation of the optic nerve fibers with oxygen.
Surgical treatment is aimed at removing formations that put pressure on the optic nerve. During the operation, the surgeon can implant biogenic materials into the patient, which will help improve blood circulation in the eye and in the atrophied nerve, in particular.